��Ŀ����
8����ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����HNO3��H2CO3��H2SiO3���ѧʽ����
��2��������Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ�ֻ�����û������������ӻ��������ӻ�������ۻ���������˻�����ĵ���ʽΪ
 ����ѧ�����������Ӽ������Լ�������Ӽ��������Լ����Ǽ��Լ�������
����ѧ�����������Ӽ������Լ�������Ӽ��������Լ����Ǽ��Լ���������3���õ���ʽ��ʾ������γɻ�����Ĺ��̣�
 ��
����4��0.1molNa218O2�������Ģڵ������������ȫ��Ӧ�����ù��������Ϊ10.8g
��5����ij���ʵ�һ�������ﺬ��һ����ԭ�ӣ�һ����ԭ�ӣ�һ����ԭ�Ӻ�һ����ԭ�ӣ��ĸ�ԭ�ӹ��γ�5�Թ��õ��Ӷԣ�д���˷��ӵĽṹʽH-S-C��N��H-N=C=S��
���� ��Ԫ�������ڱ���λ�ã�֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1���ǽ�����Խǿ����ۺ����������Խǿ��
��2��������Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ�ֻ�����û�����ΪNH4Cl��
��3��������γɻ�����CS2�����ڹ��ۻ����
��4��������Ӧ��2Na218O2+2CO2=2Na2C18OO2+18O2����֪���ɹ���Ϊ0.1mol�������ɵ�̼���Ƶ�Ħ������Ϊ108g/mol��
��5����ij���ʵ�һ�������ﺬ��һ��Hԭ�ӣ�һ��Cԭ�ӣ�һ��Nԭ�Ӻ�һ��Sԭ�ӣ��ĸ�ԭ�ӹ��γ�5�Թ��õ��Ӷԣ���Hԭ���γ�1�ԣ�Cԭ���γ�4�ԣ�Nԭ���γ�3�ԣ�Sԭ���γ�2�ԣ�������ΪH-S-C��N��H-N=C=S��
��� �⣺��Ԫ�������ڱ���λ�ã�֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1���ǽ�����N��C��Si���ǽ�����Խǿ����ۺ����������Խǿ�������ԣ�HNO3��H2CO3��H2SiO3��
�ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��2��������Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ�ֻ�����û�����ΪNH4Cl���������ӻ��������ʽΪ ���������Ӽ������Լ���
���������Ӽ������Լ���
�ʴ�Ϊ�����ӻ���� �����Ӽ������Լ���
�����Ӽ������Լ���
��3��������γɻ�����CS2�����ڹ��ۻ�����õ���ʽ��ʾ�γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��4��������Ӧ��2Na218O2+2CO2=2Na2C18OO2+18O2����֪���ɹ���Ϊ0.1molNa2C18OO2�������ɵ�̼���Ƶ�Ħ������Ϊ108g/mol�����ù�������Ϊ0.1mol��108g/mol=10.8g��
�ʴ�Ϊ��10.8��
��5����ij���ʵ�һ�������ﺬ��һ��Hԭ�ӣ�һ��Cԭ�ӣ�һ��Nԭ�Ӻ�һ��Sԭ�ӣ��ĸ�ԭ�ӹ��γ�5�Թ��õ��Ӷԣ���Hԭ���γ�1�ԣ�Cԭ���γ�4�ԣ�Nԭ���γ�3�ԣ�Sԭ���γ�2�ԣ��÷��ӵĽṹʽΪH-S-C��N��H-N=C=S��
�ʴ�Ϊ��H-S-C��N��H-N=C=S��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã������������ڱ��Ľṹ����4���йؼ������ⷴӦԭ���ж�̼���Ƶ���ɣ�ע���õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�| A�� | ����ʽΪC3H8��C6H14�������л���һ����Ϊͬϵ�� | |
| B�� | ����������һ����CH2ԭ���ŵĻ�����ض���Ϊͬϵ�� | |
| C�� | ͬϵ��ķ�������ֵһ�����14�������� | |
| D�� | ͬϵ��Ļ�ѧ�������ƣ�����������̼ԭ�����ĵ������ֹ����Ա仯 |
| A�� | Z����̬�⻯���ȶ���ǿ�������ķǽ�����̬�⻯�� | |
| B�� | ԭ�Ӱ뾶��Z��Y��X | |
| C�� | CaY2��ˮ����������ԭ��Ӧʱ��CaY2ֻ�������� | |
| D�� | CaX2��CaY2��CaZ23�ֻ������У��������������Ӹ����Ⱦ�Ϊ1��2 |
| A�� | ������Һ�У�Rb+��Cs+��CH3COO-��Br- | B�� | ������ˮ�� I-��NO3-��Na+��SO32- | ||
| C�� | D+��Cl-��NO3-��SiO32- | D�� | Ag+��Fe3+��Br-��SO42- |
 ��һ���¶��£���ij�ܱ�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ��ʾ�����������У���ȷ���ǣ�������
��һ���¶��£���ij�ܱ�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ��ʾ�����������У���ȷ���ǣ�������| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪM�T2N | |
| B�� | ��t1=1����Ӧ��ʼ��t1ʱ���M �ķ�Ӧ����Ϊ1mol�qL-1�qmin-1 | |
| C�� | t2ʱ���淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬ | |
| D�� | t3ʱ����Ӧ���ʵ����淴Ӧ���� |
| A�� | ϡHCl | B�� | HF�� | C�� | NaOH��Һ | D�� | Na2SiO3��Һ |
| ���ۼ� | H2���� | N2���� | NH3���� |
| ���ܣ�KJ•mo1-1�� | 436 | 945 | 391 |
��2����298Kʱ��ȡ1mol������3mol��������һ�ܱ������У��ڴ��������½��з�Ӧ�� �����Ϸų������յ�����ΪQ1����Q1Ϊ93KJ��
��3��ʵ�������У��ų������յ�����ΪQ2��Q1��Q2�Ƚϣ���ȷ����B
A��Q1��Q2B��Q1��Q2 C��Q1=Q2��
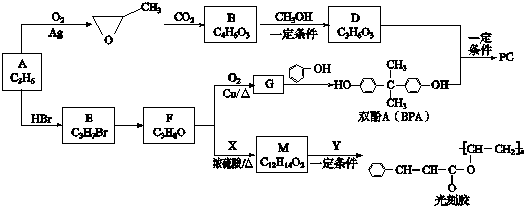
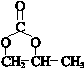 ��
��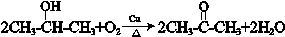 ��
��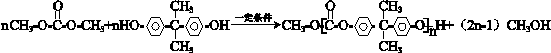 ��
��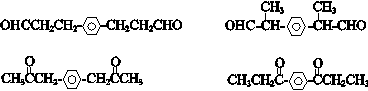 ������һ�֣���
������һ�֣���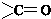 �ṹ
�ṹ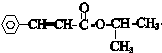 +
+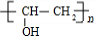 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$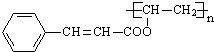 +nCH3CHOHCH3��
+nCH3CHOHCH3��