��Ŀ����
13��ij������ȤС���ԱΪ�о�����ͭ��ǿ��ķ�Ӧ����9.6��ͭ�۷�Ϊ���ȷݣ�����������һЩʵ�飬������йؼ��㣮��1��ȡ����һ����100mLһ��Ũ�ȵ����ᷴӦ����������ȫ�ܽ⣬������һ�������Ͷ���������������ڱ�״���µ����Ϊ0.896L��Ȼ��ʣ����Һϡ����1000mL�����pH=0������㣺���ɵĻ��������һ���������������Ϊ75%����Ӧǰ��������ʵ���Ũ����11.4mol/L��
��2����ȡ��һ��ͭ��Ͷ��100mLpH=0�������������Һ�У���ʹ��Ӧ�����ɺ�����һ����������448mL����״��������Ӧǰ�����Һ��������������ʵ����Ƕ��٣���д��������̣�
��3����NaOH��Һ���յ��������Ƿ�ֹNOx��Ⱦ��һ�ַ�����
ԭ��Ϊ��2NO2+2NaOH��NaNO3+NaNO2+H2O��NO+NO2+2NaOH��2NaNO2+H2O
ȡ���һ��ͭ������a mol/L������������Һ30.0ml�У���������Ļ�ԭ����ֻ��һ�������Ͷ�������������Ӧ��������ʣ��Һ��ˮϡ����1000m L�����c��NO3-��=0.200mol/L������Ӧ�����ɵĻ�������ܱ�NaOH��Һ��ȫ���գ�������a��ȡֵ��Χ��
���� n��Cu��=$\frac{9.6g}{64g/mol}$=0.15mol��
��1��n��NOx��=$\frac{0.896L}{22.4L/mol}$=0.04mol��ʣ����Һ��n��HNO3��=1mol/L��1L=1mol����NO��NO2�����ʵ����ֱ�Ϊx��y�����ת�Ƶ����غ��$\left\{\begin{array}{l}{x+y=0.04}\\{��5-2��x+y��5-4��=0.15��\frac{1}{3}��2}\end{array}\right.$��
���x=0.03mol��y=0.01mol��
��ͬ�����£���ͬ��������ʵ���֮�ȵ��������֮�ȣ�NO������������������ʵ���������
����Nԭ���غ�֪��ԭ����Һ��n��HNO3��=2[Cu��NO3��2]+n��NOx��+n��HNO3����ʣ�ࣩ=2n��Cu��+n��NOx��+n��HNO3����ʣ�ࣩ=0.15mol��$\frac{1}{3}$��2+0.04mol+1mol=1.14mol��
����c=$\frac{n}{V}$�����������ʵ���Ũ�ȣ�
��2��n��NO��=$\frac{0.448L}{22.4L/mol}$=0.02mol��ԭ�������Һ��pH=0���������������Һ��c��H+��=1mol/L��
n��H+��=0.1L��1mol/L=0.1mol��n��Cu��=0.15mol��$\frac{1}{3}$=0.05mol������3Cu+8H++2NO3-=3Cu2++2NO��+3H2O��֪�����ͭ��ȫ��Ӧ��Ҫn��H+��=$\frac{0.05mol��8}{3}$��0.13mol��0.1mol��ͭ������������ȫ��Ӧ������n��HNO3��=n��NO��=0.02mol��
ԭ����Һ��n��H+��=2n��H2SO4��+n��HNO3��=2n��H2SO4��+0.02mol=0.1mol��
��3����n��NO2��=xmol��n��NO��=ymol��
���ݵ����غ㣬��x+3y=0.05��2��
����NԪ���غ㣬��x+y=0.03a-0.2��
��֮�ã�x=��0.045a-0.35��mol��y=��0.15-0.015a��mol��
Ҫʹ���������ȫ��NaOH���գ������ɵĶ���������һ���������Ϊ��1��1�����ü����������
��� �⣺��1��n��Cu��=$\frac{9.6g}{64g/mol}$=0.15mol��n��NOx��=$\frac{0.896L}{22.4L/mol}$=0.04mol��ʣ����Һ��n��HNO3��=1mol/L��1L=1mol����NO��NO2�����ʵ����ֱ�Ϊx��y�����ת�Ƶ����غ��$\left\{\begin{array}{l}{x+y=0.04}\\{��5-2��x+y��5-4��=0.15��\frac{1}{3}��2}\end{array}\right.$��
���x=0.03mol��y=0.01mol��
��ͬ�����£���ͬ��������ʵ���֮�ȵ��������֮�ȣ�NO������������������ʵ�������Ϊ$\frac{0.03mol}{0.03mol+0.01mol}$��100%=75%��
����Nԭ���غ�֪��ԭ����Һ��n��HNO3��=2[Cu��NO3��2]+n��NOx��+n��HNO3����ʣ�ࣩ=2n��Cu��+n��NOx��+n��HNO3����ʣ�ࣩ=0.15mol��$\frac{1}{3}$��2+0.04mol+1mol=1.14mol����c=$\frac{n}{V}$��֪�������ʵ���Ũ��Ϊ$\frac{1.14mol}{0.1L}$=11.4mol/L��
�ʴ�Ϊ��75%��11.4mol/L��
��2��NO�����ʵ�����0.448L��22.4L/mol=0.02mol��Ͷ���ͭ��0.05mol�����ݷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��֪ͭ��ȫ��Ӧ��Ҫ�����ӵ����ʵ�����$\frac{8}{3}��0.05mol$����Һ����������0.1L��1.0mol/L=0.1mol����˵�������Ӳ��㣬ͭ������������ȫ��Ӧ�����ݵ�ԭ���غ��֪��������ʵ�����0.02mol������ԭ��Һ����������ʵ�����$\frac{0.1mol-0.02mol}{2}$=0.04mol��
�𣺷�Ӧǰ�����Һ��������������ʵ�����0.04mol��
��3����n��NO2��=xmol��n��NO��=ymol��
���ݵ����غ㣬��x+3y=0.05��2��
����NԪ���غ㣬��x+y=0.03a-0.2��
��֮�ã�x=��0.045a-0.35��mol��y=��0.15-0.015a��mol��
������ȫ������ԭΪNO2����ת�Ƶ�����Ŀ��ȿ�֪���ɵ�NO2���ʵ���Ϊn��NO2��=2n��Cu��=2��0.05mol=0.1mol����0.03a-0.2=0.1��a=10��
������NO��NO2��������ȫ�����գ����ɷ�Ӧ2NO2+2NaOH=NaNO3+NaNO2+H2O��NO2+NO+2NaOH=2NaNO2+H2O��֪��Ӧ����n��NO2����n��NO����
��������ʵ�����ȣ��Ҷ�Ϊxmol������ת�Ƶ�����Ŀ��ȿ�֪x+3x=0.05��2��x=0.025��
��ʱ0.03a-0.2=0.025��2��
a=8.3��
��a��8.3��
Ӧ����8.3��a��10����
��a��ȡֵ��ΧΪ8.3��a��10����
���� ���⿼��������ԭ��Ӧ�ļ��㣬Ϊ��Ƶ���㣬�������ʵ����ʡ������ķ�ӦΪ���Ĺؼ������ط�������������Ŀ��飬ע����Ӽ�ԭ���غ�Ӧ�ã���Ŀ�ѶȲ���

 һ����������ϵ�д�
һ����������ϵ�д�| A�� | ZnΪ������Ag2O������ | |
| B�� | ��ʹ�ù����У���ظ�������Һ��c��OH-��ֵ��С | |
| C�� | ��ع���ʱ��������Zn�������·����Ag2O�� | |
| D�� | ��·��ÿͨ��0.2mol���ӣ����������������ϼ���6.5g |
��������ͭ������Һ�����ᷴӦ��OH-+H+�TH2O
���Ȼ�����Һ�������ˮ��Ӧ��Al3++3NH2•H2O�TAl��OH��3��+3NH4+
��С�մ���Һ�뱥��ʯ��ˮ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O
�ܱ�������Һ��ͨ�������CO2��C6H5O-+H2O+CO2�TC6H5OH��+HCO3-��
| A�� | �١��� | B�� | �١��� | C�� | �ڡ��� | D�� | �١��� |
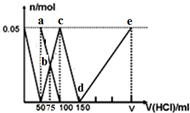
| A�� | a����Һ�У�c��HCO3-��+c��H2CO3��+c��H+��=c��OH-�� | |
| B�� | b��������Һ��������Ũ�ȴ�С˳��Ϊ��c��Cl-����c��HCO3-����c��CO32-����c��OH-�� | |
| C�� | c����Һ�У�c��Cl-��+c��HCO3-��+c��H2CO3��+c��CO32-��=1.5 mol•L-1 | |
| D�� | d��e�Ĺ�����ˮ�ĵ���̶���С |
| A�� | Ag+��NO3-��Cl-��K+ | B�� | Na+��Fe2+��Cl-��NO3- | ||
| C�� | K+��Ba2+��OH-��SO42- | D�� | Cu2+��NH4+��Br-��Cl- |
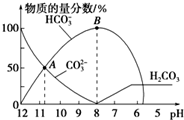
| A�� | ��A����ʾ����Һ�У�c��Na+��+c��H+��=2c��CO32-��+c��HCO3-��+c��OH-�� | |
| B�� | ��B����ʾ����Һ�У�Ũ������������Na+ | |
| C�� | ����Һ��pHΪ7ʱ����Һ�����������20mL | |
| D�� | 25��ʱ��CO32-ˮ�ⳣ��Kh=2��10-4mol•L-1������Һ��c��HCO3-����c��CO32-��=2��1ʱ����Һ��pH=10 |
| A�� | HCO3-��Ag+��NO3-��Na+ | B�� | Na+��Cl-��CO32-��NO3- | ||
| C�� | Fe3+��Na+��Cl-��SO42- | D�� | H+��Cl-��CO32-��NH4+ |
| A�� | 0.5mol H2O���е�ԭ����ĿΪ1.5NA | |
| B�� | 32g O2������ԭ����ĿΪNA | |
| C�� | 1mol H2O���е�H2O������ĿΪNA | |
| D�� | 0.5NA���������ӵ����ʵ�����0.5mol |