��Ŀ����
ij�л����A��Ϊͬ���칹�壬���ⶨ���ǵ���Է�������С��100����1mol����O2�г��ȼ�յõ������ʵ�����CO2��H2O ��g ����ͬʱ����112LO2����״�����������������½�1mol����ȫˮ���������1mol�Һ�1mol����������һ�������£������Ա�����������Ϊ�ң�
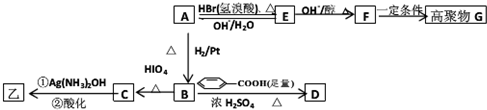
��������ײⶨ���ڼ�A�Ľṹ�ж�����C=O˫����C-O������B��HIO4���ڲ�����ʱֻ����һ�ֲ���C������Ϊ����ط�Ӧ����Ϣ��ת����ϵ��
��ROH+HBr�������ᣩ
RBr+H2O
��
��1����ȷ����д���ķ���ʽ �������ͬ�����ʵ�ͬ���칹�干�� �֣������ף���
��2��E��F �ķ�Ӧ����Ϊ ��Ӧ��
��3��A �Ľṹ��ʽΪ ��G �Ľṹ��ʽΪ ��
��4��B��D�ķ�Ӧ��ѧ����ʽΪ�� ��
��5��д��C���������½��з�Ӧ�Ļ�ѧ����ʽ�� ��

��������ײⶨ���ڼ�A�Ľṹ�ж�����C=O˫����C-O������B��HIO4���ڲ�����ʱֻ����һ�ֲ���C������Ϊ����ط�Ӧ����Ϣ��ת����ϵ��
��ROH+HBr�������ᣩ
| �� |
��

��1����ȷ����д���ķ���ʽ
��2��E��F �ķ�Ӧ����Ϊ
��3��A �Ľṹ��ʽΪ
��4��B��D�ķ�Ӧ��ѧ����ʽΪ��
��5��д��C���������½��з�Ӧ�Ļ�ѧ����ʽ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������1mol�������������¿�ˮ������1mol �Һ�1mol��������к����������ұ����Ա�����������Ϊ�ң�����Ǵ��������ᣬ�����ҷ���ס������̼ͬԭ����Ŀ������Oԭ����Ŀ����Ϊ2.1mol ����O2 �г��ȼ�յõ������ʵ�����CO2��H2O��g����˵������̼����ԭ�Ӹ�������1��2��ͬʱ����112L O2����״�������������ʵ���Ϊ
=5mol�����л���X�ķ���ʽΪCnH2nOx����n+
-
=5��������3n-x=10����Mr���ף���100�����ۿɵ�x=2��n=4�������ʽΪC4H8O2�������CH3COOCH2CH3������CH3COOH������CH3CH2OH��C��������Һ����������ӦȻ���ữ�����ᣬ��C��CH3CHO��B��HIO4���ڲ�����ʱֻ����һ�ֲ���C�������Ϣ��֪��BΪCH3CH��OH��CH��OH��CH3��A���Ϊͬ���칹�壬��A�к���̼��˫������AΪ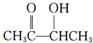 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��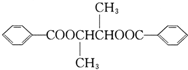 ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��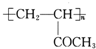 ��
��
| 112L |
| 22.4L/mol |
| 2n |
| 4 |
| x |
| 2 |
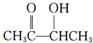 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ�� ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��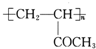 ��
�����
�⣺1mol�������������¿�ˮ������1mol �Һ�1mol��������к����������ұ����Ա�����������Ϊ�ң�����Ǵ��������ᣬ�����ҷ���ס������̼ͬԭ����Ŀ������Oԭ����Ŀ����Ϊ2.1mol ����O2 �г��ȼ�յõ������ʵ�����CO2��H2O��g����˵������̼����ԭ�Ӹ�������1��2��ͬʱ����112L O2����״�������������ʵ���Ϊ
=5mol�����л���X�ķ���ʽΪCnH2nOx����n+
-
=5��������3n-x=10����Mr���ף���100�����ۿɵ�x=2��n=4�������ʽΪC4H8O2�������CH3COOCH2CH3������CH3COOH������CH3CH2OH��C��������Һ����������ӦȻ���ữ�����ᣬ��C��CH3CHO��B��HIO4���ڲ�����ʱֻ����һ�ֲ���C�������Ϣ��֪��BΪCH3CH��OH��CH��OH��CH3��A���Ϊͬ���칹�壬��A�к���̼��˫������AΪ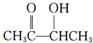 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ�� ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��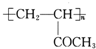 ��
��
��1��������������֪���ķ���ʽΪC4H8O2������CH3COOCH2CH3�������ͬ�����ʵ�ͬ���칹���У����������������������������������������������4�֣�
�ʴ�Ϊ��C4H8O2��4��
��2��E��F��CH3COCH��Br��CH3���������ƵĴ���Һ������ȥ��Ӧ����CH3COCH=CH2���ʴ�Ϊ����ȥ��Ӧ��
��3��������������֪��A �Ľṹ��ʽΪ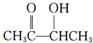 ��G �Ľṹ��ʽΪ
��G �Ľṹ��ʽΪ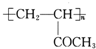 ���ʴ�Ϊ��
���ʴ�Ϊ��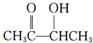 ��
��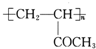 ��
��
��4��B��D�ķ�Ӧ��ѧ����ʽΪ��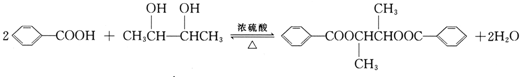 ��
��
�ʴ�Ϊ�� ��
��
��5��C���������½��з�Ӧ�Ļ�ѧ����ʽ��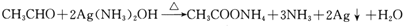 ��
��
�ʴ�Ϊ��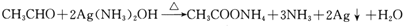 ��
��
| 112L |
| 22.4L/mol |
| 2n |
| 4 |
| x |
| 2 |
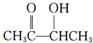 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ�� ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��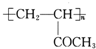 ��
����1��������������֪���ķ���ʽΪC4H8O2������CH3COOCH2CH3�������ͬ�����ʵ�ͬ���칹���У����������������������������������������������4�֣�
�ʴ�Ϊ��C4H8O2��4��
��2��E��F��CH3COCH��Br��CH3���������ƵĴ���Һ������ȥ��Ӧ����CH3COCH=CH2���ʴ�Ϊ����ȥ��Ӧ��
��3��������������֪��A �Ľṹ��ʽΪ
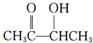 ��G �Ľṹ��ʽΪ
��G �Ľṹ��ʽΪ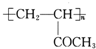 ���ʴ�Ϊ��
���ʴ�Ϊ��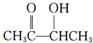 ��
��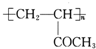 ��
����4��B��D�ķ�Ӧ��ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����5��C���������½��з�Ӧ�Ļ�ѧ����ʽ��
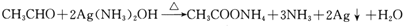 ��
���ʴ�Ϊ��
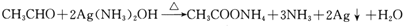 ��
��
���������⿼���л�����ƶϣ�����ȷ���Ľṹ��ʽ�ǹؼ����ٽ��ת����ϵ�з�Ӧ�����ƶϣ�ע��������Ŀ����ķ�Ӧ��Ϣ��ע������л�������ŵ������Լ�ת�����Ѷ��еȣ�

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ
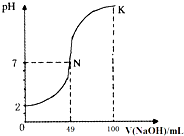 �����£���100mL0.1mol?L-1H2A����Ԫ�ᣩ��Һ����μ���0.2mol?L-1NaOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ��������й�˵����ȷ���ǣ�������
�����£���100mL0.1mol?L-1H2A����Ԫ�ᣩ��Һ����μ���0.2mol?L-1NaOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ��������й�˵����ȷ���ǣ�������| A��H2AΪ��Ԫǿ�� |
| B��K��ʱ��ˮϡ����Һ��c��H+������ |
| C��N���Ӧ��Һ�У�c��Na+��=c��A2-��+c��HA-�� |
| D��K���Ӧ��Һ������Ũ���ɴ�С��˳��Ϊ��c��A2-����c��Na+����c��OH-����c��H+�� |
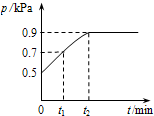 ��1.0L�ܱ������з���0.10 mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
��1.0L�ܱ������з���0.10 mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������A���ӷ�Ӧ��ʼ��t1ʱ��ƽ����Ӧ����v��X��=
| ||
| B�����¶��´˷�Ӧ��ƽ�ⳣ��K=0.32 | ||
| C�������ƽ����ϵ��Y�ĺ�������������ϵ�¶Ȼ����Z���� | ||
| D�������������䣬�ٳ���0.1 mol ����X��ƽ�������ƶ���X��ת�������� |

 ��֪�������ճ������г��������ᣮ
��֪�������ճ������г��������ᣮ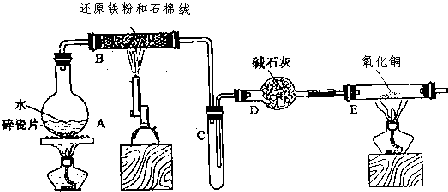
 ��ʵ�����У�����ͼ��ʾװ�ã�β������װ����ȥ����������ʵ�飬������Һ����ε��뵽���У�ʵ������Ԥ�������һ�µ��� ��������
��ʵ�����У�����ͼ��ʾװ�ã�β������װ����ȥ����������ʵ�飬������Һ����ε��뵽���У�ʵ������Ԥ�������һ�µ��� �������� 
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ� Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ����
Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ����