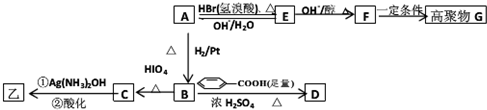
��Ŀ����
 ��֪�������ճ������г��������ᣮ
��֪�������ճ������г��������ᣮ��1����pH��ֽ�ⶨ����pH�IJ�����
��2����������pH=5�Ĵ���ϡ��Һ�У�����������c��H+���ľ�ȷֵ��
��3����0.100 0mol?L-1NaOH��Һ�ζ�20.00mLijŨ�ȵ�CH3COOH��Һ�����ֲ������£�
��ȡһ֧������ˮϴ���ļ�ʽ�ζ��ܣ����������������Һ����¼��ʼ����
������ʽ�ζ��ܷų�һ��������Һ������������ˮϴ������ƿ�У�����2�μ���
�۵ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��ѡ������ʵ������еĴ���֮��
���㣺�к͵ζ�
ר�⣺ʵ����
��������1����pH��ֽ�ⶨ��ҺpH�ķ���Ϊ���ò�����պȡ��������Һ����PH��ֽ�ϣ�Ȼ�����ֽ��ʾ����ɫ�����ɫ�����գ�����ȷ����Һ�����ȣ�
��2��������pH=5�Ĵ�����Һ��c��H+��=1��10-5 mol/L��H2O?H++OH-��Һ��c��OH-��ֻ������ˮ�ĵ��룬����c��OH-��=
���ˮ�������c��H+�����������������c��H+���ľ�ȷֵ��
��3���ٵζ���Ӧ��ϴ��������CH3COONaˮ��ʼ��ԣ�Ӧ�÷�̪��ָʾ�����۵ζ�ʱӦע����ƿ����Һ��ɫ�ı仯���к͵ζ���������Һ���õ��ձ��ͽ�ͷ�ιܣ�
��2��������pH=5�Ĵ�����Һ��c��H+��=1��10-5 mol/L��H2O?H++OH-��Һ��c��OH-��ֻ������ˮ�ĵ��룬����c��OH-��=
| KW |
| c(H+) |
��3���ٵζ���Ӧ��ϴ��������CH3COONaˮ��ʼ��ԣ�Ӧ�÷�̪��ָʾ�����۵ζ�ʱӦע����ƿ����Һ��ɫ�ı仯���к͵ζ���������Һ���õ��ձ��ͽ�ͷ�ιܣ�
���
�⣺��1��pH��ֽ�ⶨpH�ķ����ǣ���һСƬpH��ֽ���ڱ������ϣ��ò�������ͷ�ιܽ�����Һ������ֽ�ϣ��ٽ���ɫ����ֽ�����ɫ�����ն�����ֵ��
�ʴ�Ϊ�������Ӽ�ȡһС����ֽ���ڸ���ྻ�ı��������Ƭ�ϣ��ò�����պȡ����Һ������ֽ���в����۲���ɫ�仯�������ɫ���ȶԶ�����
��2��������pHΪ5�Ĵ�����c��H+��=1��10-5mol?L-1�������������Դ���ĵ����ˮ�ĵ��룬H2O?H++OH-��Һ��c��OH-��ֻ������ˮ�ĵ��룬
c��OH-��=
=
=1��10-9 mol?L-1������Һ��c��OH-��ˮ=c��H+��ˮ=1��10-9 mol?L-1�����Դ���������c��H+��Ϊ��
������c��H+��=1��10-5mol?L-1����ȥˮ�������c��H+����ֵ=��10-5-10-9��mol/L��
�ʴ�Ϊ��10-5-10-9��
��3���ٵζ���Ӧ��ϴ��������CH3COONaˮ��ʼ��ԣ�Ӧ�÷�̪��ָʾ�����۵ζ�ʱӦע����ƿ����Һ��ɫ�ı仯���к͵ζ���������Һ���õ��ձ��ͽ�ͷ�ιܣ�
�ʴ�Ϊ���٢ڢۣ��ձ������ձ��ͽ�ͷ�ιܣ���
�ʴ�Ϊ�������Ӽ�ȡһС����ֽ���ڸ���ྻ�ı��������Ƭ�ϣ��ò�����պȡ����Һ������ֽ���в����۲���ɫ�仯�������ɫ���ȶԶ�����
��2��������pHΪ5�Ĵ�����c��H+��=1��10-5mol?L-1�������������Դ���ĵ����ˮ�ĵ��룬H2O?H++OH-��Һ��c��OH-��ֻ������ˮ�ĵ��룬
c��OH-��=
| KW |
| c(H+) |
| 1��10-14 |
| 10-5 |
������c��H+��=1��10-5mol?L-1����ȥˮ�������c��H+����ֵ=��10-5-10-9��mol/L��
�ʴ�Ϊ��10-5-10-9��
��3���ٵζ���Ӧ��ϴ��������CH3COONaˮ��ʼ��ԣ�Ӧ�÷�̪��ָʾ�����۵ζ�ʱӦע����ƿ����Һ��ɫ�ı仯���к͵ζ���������Һ���õ��ձ��ͽ�ͷ�ιܣ�
�ʴ�Ϊ���٢ڢۣ��ձ������ձ��ͽ�ͷ�ιܣ���
���������⿼��ˮ�ĵ��롢�к͵ζ���������������һ�����ʵ���Ũ�ȵ���Һ��������Ŀ�Ѷ��еȣ������漰����֪ʶ��϶࣬ע��������������һ�����ʵ���Ũ�ȵ���Һ���衢�к͵ζ�����������

��ϰ��ϵ�д�
�����Ŀ
���й���������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ˵����ȷ���ǣ�������
| A�����Ϸ�Ӧ������������ԭ��Ӧ |
| B���е������ɵķֽⷴӦһ����������ԭ��Ӧ |
| C����һЩ�û���Ӧ����������ԭ��Ӧ |
| D������Ԫ�ز���ĸ��ֽⷴӦ��������ԭ��Ӧ |
���и������ʾ����ڹ�������Ʒ���ǣ�������
| A���մɡ�ˮ�� |
| B�������衢���� |
| C��ʯ�ࡢƯ�� |
| D��ˮ���������ȼ� |
2012��2��6�գ���������������Ϫ�εĵ�ϼ��·������Ӧ����·������������β���ش�Ľ�ͨ�¹ʣ��������������ַ�ɢϵ��������
| A������Һ | B����Һ |
| C������ | D������Һ |
ij�����ɳ�����һ�ֻ����������ɣ����ⶨ����ֻ����̼��������Ԫ�أ�̼����Ԫ�ص�������Ϊ3��8������ڸ������˵����ȷ���ǣ�������
| A��������һ���Ǵ����� |
| B��������һ����CO��CO2�Ļ���� |
| C�����������������������ֻ��2�� |
| D���������������������3����� |



 ��50mL0.5mol/L��������50mL0.55mol/L��������������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.5mol/L��������50mL0.55mol/L��������������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺