��Ŀ����
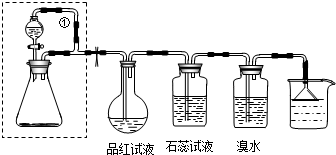
 Ϊ̽��SO2����Ļ�ԭ��ij��ȤС���������װ��ͼ��
Ϊ̽��SO2����Ļ�ԭ��ij��ȤС���������װ��ͼ����1��֤��SO2��Fe3+������Ӧ������Ϊ
��2��ʵ���������SO2���������Ũ�����ͭ��Ӧ����ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ
��3��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ����ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ������죬��ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ����
�����������������
���㣺̽������������ˮ��Ʒ����Һ�ķ�Ӧ
ר�⣺ʵ����
��������1������SO2��Fe3+������Ӧ�ķ�Ӧ�����������ʷ������
��2��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ��
��3����������Fe2+���л�ԭ�ԣ����������ܷ���������ԭ��Ӧ����ʹ���������Һ�������ԣ�
��2��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ��
��3����������Fe2+���л�ԭ�ԣ����������ܷ���������ԭ��Ӧ����ʹ���������Һ�������ԣ�
���
�⣺��1��SO2��Fe3+������ӦSO2+2Fe3++2H2O=SO42-+2Fe2++4H+��ԭ��Ϊ��ɫ��������Һ������������ԭ��dz��ɫ������������Һ��
�ʴ�Ϊ��A����Һ�ɻ�ɫ��Ϊdz��ɫ��
��2��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ����Ӧ�ķ���ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2����
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2����
��3�����������л�ԭ�ԣ����������ǿ�����ԣ������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ���������������в��������Ǣ٣�
�ʴ�Ϊ���٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
�ʴ�Ϊ��A����Һ�ɻ�ɫ��Ϊdz��ɫ��
��2��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ����Ӧ�ķ���ʽΪ��Cu+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��3�����������л�ԭ�ԣ����������ǿ�����ԣ������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ���������������в��������Ǣ٣�
�ʴ�Ϊ���٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
���������⿼����Ƕ�����������ʼ�ʵ�����Ʒ���ע����������л�ԭ�ԣ���ǿ����������������ԭ��Ӧ��������Ư���ԣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
ij�Ͻ𣨽���ͭ��������ͭ���������ʵ���֮��Ϊy mol������Cu�����ʵ�������Ϊa������ȫ��Ͷ��50mL b mol?L-1��������Һ�У�����ʹ���ַ�Ӧ������NO��Ψһ�Ļ�ԭ���������˵����ȷ���ǣ�������
| A����������ʣ�࣬����Һ���ٵ�����������ֿ�ʼ�ܽ� | ||
| B��������ȫ���ܽ⣬����Һ��һ������Fe3+ | ||
| C��������ȫ���ܽ⣬�Ҳ���336mL���壨��״��������b=0.3 | ||
D������Һ�н�������ֻ��Fe3+��Cu2+ʱ����a��b�Ĺ�ϵΪ��b��80y��1-
|
���й���ͭ��˵����ȷ���ǣ�������
| A��ͭ�ڸ���Ŀ����к��ڳ�ʪ�Ŀ����д��ڵ���̬��ͬ |
| B����ͬ������ͭ�ֱ�������������������ȫ��Ӧ��ʧȥ�ĵ�������ͬ |
| C����ͬ���ʵ�����Ũ����ֱ���������ͭ������ͭ��Ӧ����������ͭ������ͬ |
| D����ͬ������ͭ�ֱ���������ϡ���ᡢŨ������ȫ��Ӧ��������������ʵ�����ͬ |
 �����£�ȡpH=2�����ֶ�Ԫ��H2A��H2B��1ml���ֱ��ˮϡ�ͣ����PH�仯���ˮϡ�ͱ�������ͼ��ʾ�仯���������й�������ȷ���ǣ�������
�����£�ȡpH=2�����ֶ�Ԫ��H2A��H2B��1ml���ֱ��ˮϡ�ͣ����PH�仯���ˮϡ�ͱ�������ͼ��ʾ�仯���������й�������ȷ���ǣ�������| A��H2AΪ��Ԫ���� |
| B��PH=4��NaHAˮ��Һ������Ũ�ȴ�СΪc��Na+����c��HA-����c��A2-����c��H2A����c��OH-�� |
| C����NaHA��NaHB�Ļ����Һ�У�����Ũ�ȴ�СΪc��Na+��=c��A2-��+c��HB-��+c��H2B��+c��B2-�� |
| D��Na2B��ˮ��Һ�У�����Ũ�ȴ�СΪc��Na+����c��B2-����c��OH-����c��H+�� |
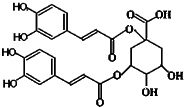 ������һ���½ṹ���͵Ŀ������ײ����Ϳ����̲������Ļ������ṹ��ͼ��ʾ���й����ص�˵����ȷ���ǣ����ĸ���ͬ���ŵ�̼��������̼����������
������һ���½ṹ���͵Ŀ������ײ����Ϳ����̲������Ļ������ṹ��ͼ��ʾ���й����ص�˵����ȷ���ǣ����ĸ���ͬ���ŵ�̼��������̼����������| A�������к���6������̼ԭ�� |
| B��һ���������ܷ���������Ӧ����ȥ��Ӧ |
| C���������Ȼ�����Һ������ɫ��Ӧ |
| D��1 mol����������11 mol NaOH��Ӧ |
������������ȷ���ǣ�������
| A���Ҵ��ͱ�������Ϊͬϵ�� |
| B��16gCH4��18gNH4+��������������� |
| C��32S��33S�ĺ����������ȣ�����ͬһ�ֺ��� |
| D��������ȣ�������ȵ�CO��C2H4�ķ�������� |