��Ŀ����
9�������ж���ȷ���ǣ�������| A�� | ͬ�����ͬŨ�ȵ�NaF��NaCl��Һ�У���������������N��NaCl����N��NaF����ͬ���ͬŨ�ȵ�Na2CO3��Na2SO4��Һ�У���������������N��Na2CO3����N��Na2SO4�� | |||||||||||
| B�� | ������ij�¶��¸����ᡢ���ᡢ����������ڱ������еĵ��볣�������ڱ�����������ĵ��뷽��ʽ�ɱ�ʾΪH2SO4?2H++SO42-
| |||||||||||
| C�� | ��֪����HF��CH3COOH��pH��ȵ�NaF��CH3COOK��Һ�У�[c��Na+��-c��F-��]��[c��K+��-c��CH3COO-��] | |||||||||||
| D�� | ƽ����ϵCaCO3��s��?CaO��s��+CO2�н�����̼��ơ������Ƽ�������̼���壮ijʱ�̣������¶Ȳ��䣬�����������СΪԭ����һ�벢���ֲ��䣬�������̼Ũ������ |
���� A������F-��CO32-��ˮ�Ⲣ��ϵ���غ���������
B�����ڵ���ƽ������ڵ���ʱ�Dz���ȫ����ģ��Ҷ�Ԫ���Ƿֲ�����ģ�
C��NaF��Һ��c��Na+��-c��F-����CH3COOK��Һ��c��K+��-c��CH3COO-��������c��OH-��-c��H+����
D���¶Ȳ��䣬K=c��CO2����ƽ�ⳣ�������¶��й����жϣ�
��� �⣺A�����ݵ���غ��֪NaF��aq����c��Na+��+c��H+��=c��OH-��+c��F-��������NaF��Һ��������Ũ��Ϊ2[c��Na+��+c��H+��]�����ݵ���غ��֪�Ȼ�����Һ��������Ũ��ҲΪ2[c��Na+��+c��H+��]��NaF����ˮ�⣬��Һ��F-ˮ�⣬��Һ�ʼ��ԣ�����c��OH-����c��H+���������ʵ���Ũ�ȵ�NaNaF��aq����NaCl��aq���У�NaF��Һ��c��H+��С��NaCl��aq����c��H+��������Һ��c��Na+����ͬ�����ԣ�����������ʵ���Ũ�ȵ�NaF��aq����NaCl��aq���������������٣���������NaCl��NaF��
CO32-�ĵ�һ��ˮ��CO32-+H2O?HCO3-+OH-�ᵼ��������Ŀ�����࣬��ͬ���ͬŨ�ȵ�Na2CO3��Na2SO4��Һ�У���������������N��Na2CO3����N��Na2SO4������A��ȷ��
B���ڱ�������������ڵ���ƽ�⣬��������뷽��ʽΪH2SO4?H++HSO4-����B����
C��NaF��Һ��c��Na+��-c��F-����CH3COOK��Һ��c��K+��-c��CH3COO-��������c��OH-��-c��H+����������Һ�е�pH��ȣ���c��H+����ȣ�c��OH-��Ҳ��ȣ�������Һ�е�[c��OH-��-c��H+��]��ȣ���[c��Na+��-c��F-��]=[c��K+��-c��CH3COO-��]����C����
D��CaCO3��s��?CaO��s��+CO2��g����K=c��CO2������С�����ƽ�������ƶ�������Ϊ�¶�û�䣬��K=c��CO2�����䣬����CO2Ũ�Ȳ��䣬��D����
��ѡA��
���� ������Ҫ������ƽ���ƶ�����ƽ�ⳣ���ı���ʽ����Ӱ�����أ��ѶȲ���ץסƽ�ⳣ�������¶��йؼ��ɽ��⣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | C3H8O | B�� | C4H10O | C�� | C4H8Cl2 | D�� | C5H11Cl |

| A�� | ͼ1��ʾװ�ÿ��Ʊ����������� | |
| B�� | ͼ2��ʾװ�ÿɵ��ʳ��ˮ��������Cu��OH��2 | |
| C�� | ͼ3��ʾװ�ÿ���֤������������ˮ | |
| D�� | ͼ4��ʾװ������Cu��Ũ���ᷴӦ��ȡ������SO2���� |
| A�� | ��Һ����ʱ��Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| B�� | �������ķ���ʹNaCl����Һ������ʱ��Ӧ����������NaCl��ҺС��������� | |
| C�� | ��������ķ��������Ҵ���ˮ�ķ���ʱ���¶ȼ�ˮ����Ӧ����Һ��������ȷ�ⶨ�¶� | |
| D�� | ��ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
| A�� | ����$��_{ˮԡ����}^{������Һ}$�����������ۣ���������ȩ�� | |
| B�� | ij±��������$��_{����}^{����������Һ}$$\stackrel{������������Һ}{��}$���յij������ǰ�ɫ�����ۣ���±�����в�����ԭ�� | |
| C�� | ij��Һ$\stackrel{��ƿ��}{��}$ð�Ű���$\stackrel{��պ��Ũ��ˮ������}{��}$�����������̣����ۣ�����ҺΪŨ���� | |
| D�� | ��ɫ��Һ$\stackrel{��ɫ��Ӧ}{��}$�ʻ�ɫ�����ۣ�����Һһ��������Ԫ�� |
| A�� | 3��3-�������� 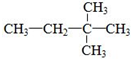 | B�� | l-��ϩ��CH2=CH-CH2-CH3 | ||
| C�� | ����ױ���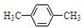 | D�� | ���״��� |
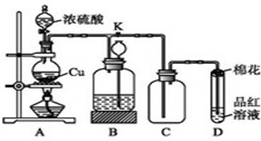 ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�飮 ��ͬѧȡag Cu Ƭ��12ml 18mol/LŨH2SO4����Բ����ƿ�м��ȣ�ֱ����Ӧ��ϣ��������ƿ�л���һ������H2SO4��Cuʣ�࣮
ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�飮 ��ͬѧȡag Cu Ƭ��12ml 18mol/LŨH2SO4����Բ����ƿ�м��ȣ�ֱ����Ӧ��ϣ��������ƿ�л���һ������H2SO4��Cuʣ�࣮