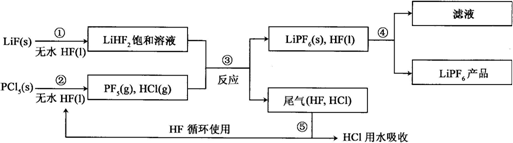
��Ŀ����
�ɼ������ӻ�������ɵĻ����������������е������֣�K+��Cl-��NH
��Mg2+��CO
��Ba2+��SO
�����û��������ˮ��ó�����Һ����ȡ3��100mL����Һ��
���������ʵ�飺
�Իش��������⣺
��1������ʵ��1��Cl-�Ƿ���ڵ��ж��� ���һ�����ڡ�����һ�������ڡ�����ȷ��������
��2��д��ʵ��3�еĿո�ʵ����� ��
��3������ʵ��1��3�жϻ������һ�������ڵ������� ��
��4����ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ�ȷֱ�Ϊ
��5��K+�Ƿ���ڣ� ���һ�����ڡ�����һ�������ڡ�����ȷ�����������������ʵ�鷽��֤�� ��
+ 4 |
2- 3 |
2- 4 |
���������ʵ�飺
| ʵ����� | ʵ������ | ʵ���� |
| 1 | ����AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ��������BaCl2��Һ����Ӧ����С������������������м�����ϡ���ᡢȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������2.33g |
��1������ʵ��1��Cl-�Ƿ���ڵ��ж���
��2��д��ʵ��3�еĿո�ʵ�����
��3������ʵ��1��3�жϻ������һ�������ڵ�������
��4����ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ�ȷֱ�Ϊ
��5��K+�Ƿ���ڣ�
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
������1 ��AgNO3��Һ�а�ɫ�������ɣ�˵����Һ�п�����Cl-��SO42-��CO32-��
2 ������NaOH��Һ�����ȣ��ռ�������1.12L��˵����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ0.05mol��
3 ������BaC12��Һʱ����Һ�г��������������п���Ϊ���ᱵ��̼�ᱵ�������߶��У����Ƴ�ԭ��Һһ��û��Ba2+��
�����ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ����������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬��ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����SO42-��CO32-��CO32-��Mg2+��Ba2+���ܹ��棬˵���������һ��û��Mg2+��Ba2+��2.33gΪ���ᱵ���������������ӵ����ʵ���Ϊ0.01mol����̼�ᱵ������Ϊ3.94g�������̼������ӵ����ʵ���Ϊ0.01mol�������������ӵ���غ㣬笠����ӵ����ʵ���Ϊ0.05mol����������ӵ����ʵ���Ϊ0.01mol��̼������ӵ����ʵ���Ϊ0.02mol����֪�û����һ������K+��Cl-�Ƿ���ڲ����ж����ݴ˽��н��
2 ������NaOH��Һ�����ȣ��ռ�������1.12L��˵����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ0.05mol��
3 ������BaC12��Һʱ����Һ�г��������������п���Ϊ���ᱵ��̼�ᱵ�������߶��У����Ƴ�ԭ��Һһ��û��Ba2+��
�����ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ����������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬��ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����SO42-��CO32-��CO32-��Mg2+��Ba2+���ܹ��棬˵���������һ��û��Mg2+��Ba2+��2.33gΪ���ᱵ���������������ӵ����ʵ���Ϊ0.01mol����̼�ᱵ������Ϊ3.94g�������̼������ӵ����ʵ���Ϊ0.01mol�������������ӵ���غ㣬笠����ӵ����ʵ���Ϊ0.05mol����������ӵ����ʵ���Ϊ0.01mol��̼������ӵ����ʵ���Ϊ0.02mol����֪�û����һ������K+��Cl-�Ƿ���ڲ����ж����ݴ˽��н��
���
�⣺����1��֪����Һ�п�����Cl-��SO42-��CO32-��
����2��֪����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ��
=0.05mol��
����3��֪�������п���Ϊ���ᱵ��̼�ᱵ�������߶����ڣ��������ӹ�������ж�ԭ��Һһ��û��Ba2+��
������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬��ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����SO42-��CO32-��CO32-��Mg2+���ܹ��棬˵���������һ��û��Mg2+��2.33gΪ���ᱵ�������SO42-�����ʵ���Ϊ��
=0.01mol����BaCO3������Ϊ��6.27g-2.33g=3.94g�������CO32-�����ʵ���Ϊ��
=0.02mol�������������ӵ���غ㣬笠����ӵ����ʵ���Ϊ0.05mol����������ӵ����ʵ���Ϊ0.01mol��̼������ӵ����ʵ���Ϊ0.02ol����֪�û����һ������K+��Cl-�Ƿ���ڲ����ж���
��1���������Ϸ�����֪�����ж��Ƿ���������ӣ�
�ʴ�Ϊ������ȷ����
��2������Һ�з�����������������Ϊ���ˣ�Ϊ�˼�С����Ҫ�Գ�������ϴ�ӣ�
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
��3���������Ϸ�����֪����Һ�д���̼������Ӻ���������ӣ���һ������Mg2+��Ba2+��
�ʴ�Ϊ��Mg2+��Ba2+��
��4�����ݷ�����֪����Һ��һ�����ڵ�������ΪSO42-��CO32-�������ӵ����ʵ���Ϊ��n�� CO32-��=0.02mol��n��SO42-��=0.01mol����Һ�����Ϊ0.1L�������ӵ�Ũ��Ϊ��c�� CO32-��=
=0.2mol/L��c��SO42-��=
=0.1mol/L��
�ʴ�Ϊ��c�� CO32-��=0.2mol/L��c��SO42-��=0.1mol/L��
��5�����ݵ���غ��֪����Һ��һ�����ڼ����ӣ�ͨ��������ɫ��Ӧ��������ӣ��۲�ʱ��Ҫ����ɫ�ܲ��������������ɫ��֤�����м����ӣ�
�ʴ�Ϊ��һ�����ڣ� ������ɫ��Ӧ��������ɫ�ܲ����������ɫ��֤�����м����ӣ�
����2��֪����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ��
| 1.12L |
| 22.4L/mol |
����3��֪�������п���Ϊ���ᱵ��̼�ᱵ�������߶����ڣ��������ӹ�������ж�ԭ��Һһ��û��Ba2+��
������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬��ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����SO42-��CO32-��CO32-��Mg2+���ܹ��棬˵���������һ��û��Mg2+��2.33gΪ���ᱵ�������SO42-�����ʵ���Ϊ��
| 2.33g |
| 233g/mol |
| 3.94g |
| 197g/mol |
��1���������Ϸ�����֪�����ж��Ƿ���������ӣ�
�ʴ�Ϊ������ȷ����
��2������Һ�з�����������������Ϊ���ˣ�Ϊ�˼�С����Ҫ�Գ�������ϴ�ӣ�
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
��3���������Ϸ�����֪����Һ�д���̼������Ӻ���������ӣ���һ������Mg2+��Ba2+��
�ʴ�Ϊ��Mg2+��Ba2+��
��4�����ݷ�����֪����Һ��һ�����ڵ�������ΪSO42-��CO32-�������ӵ����ʵ���Ϊ��n�� CO32-��=0.02mol��n��SO42-��=0.01mol����Һ�����Ϊ0.1L�������ӵ�Ũ��Ϊ��c�� CO32-��=
| 0.02mol |
| 0.1L |
| 0.01mol |
| 0.1L |
�ʴ�Ϊ��c�� CO32-��=0.2mol/L��c��SO42-��=0.1mol/L��
��5�����ݵ���غ��֪����Һ��һ�����ڼ����ӣ�ͨ��������ɫ��Ӧ��������ӣ��۲�ʱ��Ҫ����ɫ�ܲ��������������ɫ��֤�����м����ӣ�
�ʴ�Ϊ��һ�����ڣ� ������ɫ��Ӧ��������ɫ�ܲ����������ɫ��֤�����м����ӣ�
���������⿼���˳������ӵļ��鷽�������ӹ���֪ʶ��Ӧ�ã���Ŀ�Ѷ��еȣ�ע���������ӷ�Ӧ�����������������ӵķ�Ӧ�����ܹ��������ӹ��桢���ӷ�Ӧ������ȷ�ж����Ӵ��������

��ϰ��ϵ�д�
�����Ŀ
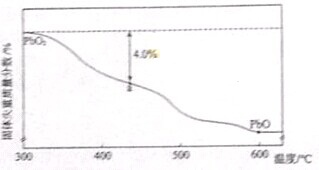
�����£���1mol��CuSO4?5H2O��s������ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1����1mol��CuSO4��s������ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4?5H2O���ȷֽ�Ļ�ѧ����ʽΪCuSO4?5H2O��s��
CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ�������
| ||
| A����H2����H3 |
| B����H1����H3 |
| C����H1+��H3=��H2 |
| D����H1+��H2����H3 |
����������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�ӵ����������������ڲ��������2����Y�ǵؿ��к�����ߵ�Ԫ�أ�Z2+��Y2-������ͬ�ĵ��Ӳ�ṹ��W��Xͬ���壬����˵����ȷ���ǣ�������
| A��ԭ�Ӱ뾶�Ĵ�С˳��r��W����r��Z����r��Y����r��X�� |
| B��Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ |
| C��X������������Ӧ��ˮ��������Ա�W���� |
| D��Y����̬���⻯������ȶ��Ա�W��ǿ |
Ԫ�������ɽ�ʾԪ�ؼ�ĵݱ���ɣ��������������еݱ��ԣ����еݱ������ȷ���ǣ�������
| A���ڶ�����Ԫ���⻯���ȶ��Ե�˳���ǣ�HF��H2O�����Ե�������Ԫ���⻯���ȶ��Ե�˳��Ҳ�ǣ�HCl��H2S |
| B��IVA��Ԫ���⻯���۵�˳���ǣ�SiH4��CH4������VA��Ԫ���⻯���۵�˳��Ҳ�ǣ�PH3��NH3 |
| C����A��Ԫ�صķǽ������ǣ�F��Cl�����Ԣ�A��Ԫ���⻯�������Ҳ�ǣ�HF��HCl |
| D��þ�������ã���ҵ���õ�������������Ʊ��������Թ�ҵ��Ҳ�õ����������þ�Ʊ�þ |