��Ŀ����
LiPF6������ӵ���й㷺Ӧ�õĵ���ʣ�ij������LiF��PCl3Ϊԭ�ϡ����·�Ӧ�Ʊ�LiPF6�����������£�
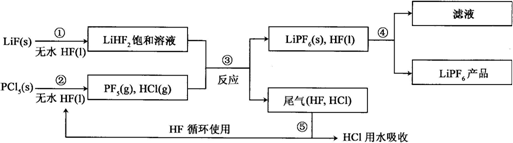
��֪��HCl�ķе���-85.0�棬HF�ķе���19.5�森
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� ���û�ѧ����ʽ��ʾ������ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ mol���ú�w��n�Ĵ���ʽ��ʾ����

��֪��HCl�ķе���-85.0�棬HF�ķе���19.5�森
��1���ڢٲ���Ӧ����ˮHF��������
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��
��3���ڢܲ�������õķ�����
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ
���㣺���⼯��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������,�绯ѧר��
��������1��������Ŀ�е����̿��Կ���������+Һ���������+������Һ��������ˮHF�������Ƿ�Ӧ����ܼ�����������Ҫ�ɷ��к��ж������裬�ܺ�HF������Ӧ��HF�������ᣬ��������������Һ����ȥ��
��2������Ԫ����ɿ�֪��������ֱ���H3PO4��HF��
��3������Һ��Ͳ�����Һ�������ù��˷��룬HF���Ӽ京��������е����HCl���ɲ������������룻
��4������Li�غ�������غ���㣮
��2������Ԫ����ɿ�֪��������ֱ���H3PO4��HF��
��3������Һ��Ͳ�����Һ�������ù��˷��룬HF���Ӽ京��������е����HCl���ɲ������������룻
��4������Li�غ�������غ���㣮
���
�⣺��1��������Ŀ�е����̿��Կ���������+Һ���������+������Һ��������ˮHF�������Ƿ�Ӧ����ܼ�����������Ҫ�ɷ��к��ж������裬�ܺ�HF������Ӧ����ѧ����ʽΪSiO2+4HF�TSiF4��+2H2O��HF�������ᣬ��������������Һ����ȥ������2%��NaHCO3��Һ����
�ʴ�Ϊ����Ӧ��ܼ���SiO2+4HF�TSiF4��+2H2O��NaHCO3��
��2��������Ŀ�е���Ϣ��PF5����ˮ�⣬�����Ϊ�����ᡱ�������Ԫ����ɿ�֪��������ֱ���H3PO4��HF�����Է�Ӧ�ķ���ʽΪPF5+4H2O�TH3PO4+5HF��
�ʴ�Ϊ��PF5+4H2O�TH3PO4+5HF��
��3���ڢܲ�������ǹ��壨LiPF4��s������Һ�壨HF��l���������Բ��ù��˵ķ���������β����HF��HCl���������ö��߷е�IJ��죨HF����֮�������������з��룬���Բ�����������
�ʴ�Ϊ�����ˣ�������
��4����LiPF6Ϊxmol��LiFΪymol������Li�غ㣬��x+y=n�����������غ���152x+26y=w�����x=
mol��
�ʴ�Ϊ��
��
�ʴ�Ϊ����Ӧ��ܼ���SiO2+4HF�TSiF4��+2H2O��NaHCO3��
��2��������Ŀ�е���Ϣ��PF5����ˮ�⣬�����Ϊ�����ᡱ�������Ԫ����ɿ�֪��������ֱ���H3PO4��HF�����Է�Ӧ�ķ���ʽΪPF5+4H2O�TH3PO4+5HF��
�ʴ�Ϊ��PF5+4H2O�TH3PO4+5HF��
��3���ڢܲ�������ǹ��壨LiPF4��s������Һ�壨HF��l���������Բ��ù��˵ķ���������β����HF��HCl���������ö��߷е�IJ��죨HF����֮�������������з��룬���Բ�����������
�ʴ�Ϊ�����ˣ�������
��4����LiPF6Ϊxmol��LiFΪymol������Li�غ㣬��x+y=n�����������غ���152x+26y=w�����x=
| w-26n |
| 126 |
�ʴ�Ϊ��
| w-26n |
| 126 |
����������Ϊ����ѧ�����⣬�����Ǹ߿��еij������ͣ������е��Ѷȵ����⣮�����ۺ���ǿ���漰�����ƶϡ������ᴿ������ʽ��д����ѧ�����֪ʶ��ע�����������Ϣ��ʹ�ã����ض�ѧ��ʵ�������������ͽ��ⷽ����ָ��������������ѧ���淶���Ͻ���ʵ����ƺ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 һ��̫���ܵ�صĹ���ԭ��ʾ��ͼ��ͼ��ʾ�������Ϊ���軯��K3[Fe��CN��6]�������軯��K4[Fe��CN��6]�Ļ����Һ������˵������ȷ���ǣ�������
һ��̫���ܵ�صĹ���ԭ��ʾ��ͼ��ͼ��ʾ�������Ϊ���軯��K3[Fe��CN��6]�������軯��K4[Fe��CN��6]�Ļ����Һ������˵������ȷ���ǣ�������| A��K+�������b |
| B������a���淢����Ӧ��Fe��CN��64--e-�TFe��CN��63- |
| C��Fe��CN��63-�ڴ���b���汻���� |
| D���������Һ��Fe��CN��63-��Fe��CN��64-Ũ�Ȼ������ֲ��� |
��a mol������b mol��ϩ��ϣ���һ��������ʹ���Dz��ַ�Ӧ����c mol���飬����Ӧ��Ļ��������ȫȼ�գ��������������ʵ���Ϊ��������
| A����3b+0.5a��mol |
| B����4b+0.5a��mol |
| C����3b+1.5a��mol |
| D�����ж� |

 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺