��Ŀ����
12������һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶã����������Ҫ�ɷ���Al2O3����������SiO2��Fe2O3����ҵ�ϴ�����������ȡ���ɲ�����ͼ�������̣�Ϊ��ת����ȫ��ÿһ�������Լ��������
��1��ԭ��A�����������ᣬԭ��B�Ļ�ѧʽNaOH��
��2��������Һ1��Fe3+���ӵ�������Լ�ΪKSCN��Һ��
��3������ڷ�Ӧ�����ӷ���ʽH++OH-=H2O��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��4������۷�Ӧ�����ӷ���ʽAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��5����ѭ��ʹ�õ�ԭ�ϵĻ�ѧʽCaCO3��NaOH��
���� ʯ��ʯ���յõ�CaO��CO2������Һ2��ͨ��CO2���õ����������ȳ����õ�����������ó���ΪAl��OH��3��CO2ͨ��ƫ��������Һ�еõ�Al��OH��3������ԭ��BΪǿ����Һ��AΪ������Һ���������м�������HCl��Al2O3��Fe2O3��ϡ���ᷴӦ����AlCl3��FeCl3��SiO2������ϡ���ᣬȻ����ù��˷����õ�����SiO2����Һ1�к���HCl��AlCl3��FeCl3������Һ�м�������NaOH��Һ��AlCl3��FeCl3�ֱ�����NaAlO2��Fe��OH��3�����ù��˷����õ�����Fe��OH��3����Һ2�к���NaAlO2����NaAlO2ͨ������Ķ�����̼������Al��OH��3��NaHCO3�����˵õ�����Һ3������ΪNaHCO3����Һ3�м��������Ƶ�̼��Ƴ���������������Һ��̼��ƺ��������ƶ�������ѭ�����ã��ݴ˴��⣮
��� �⣺ʯ��ʯ���յõ�CaO��CO2������Һ2��ͨ��CO2���õ����������ȳ����õ�����������ó���ΪAl��OH��3��CO2ͨ��ƫ��������Һ�еõ�Al��OH��3������ԭ��BΪǿ����Һ��AΪ������Һ���������м�������HCl��Al2O3��Fe2O3��ϡ���ᷴӦ����AlCl3��FeCl3��SiO2������ϡ���ᣬȻ����ù��˷����õ�����SiO2����Һ1�к���HCl��AlCl3��FeCl3������Һ�м�������NaOH��Һ��AlCl3��FeCl3�ֱ�����NaAlO2��Fe��OH��3�����ù��˷����õ�����Fe��OH��3����Һ2�к���NaAlO2����NaAlO2ͨ������Ķ�����̼������Al��OH��3��NaHCO3�����˵õ�����Һ3������ΪNaHCO3����Һ3�м��������Ƶ�̼��Ƴ���������������Һ��̼��ƺ��������ƶ�������ѭ�����ã�
��1����������ķ�����֪��ԭ��A�����������ᣬԭ��B�Ļ�ѧʽΪNaOH���ʴ�Ϊ�����NaOH��
��2��KSCN��Һ��Fe3+���ӻ�ʹ��Һ��Ѫ��ɫ�����Լ�����Һ1��Fe3+���ӵ�������Լ�ΪKSCN��Һ��
�ʴ�Ϊ��KSCN��Һ��
��3����Һ1����HCl��AlCl3��FeCl3��Ҫ�����Թ���ԭ��B��ԭ��B�Ļ�ѧʽ��NaOH����������ӡ������Ӻ����������ӷ�Ӧ�����ӷ�Ӧ����ʽΪH++OH-=H2O��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��H++OH-=H2O��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��4����Һ�ں���AlO2-�������ͨ�������̼����AlO2-��Ӧ���ɵij���Ϊ������������Ӧ�����ӷ���ʽΪ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��5����������ķ�����֪����ѭ��ʹ�õ�ԭ��ΪCaCO3��NaOH���ʴ�Ϊ��CaCO3��NaOH��
���� ���⿼�����ʷ�����ᴿ��Ϊ��Ƶ���㣬��ȷ���ʵ������ǽⱾ��ؼ���֪������ͼ�з����ķ�Ӧ��������������Һ�е����ʣ�ע�������������ԣ�ע����Һ1�л�����HCl����Һ2�л�����NaOH��


ƽ���ƶ������أ��������¶� �ڽ����¶� ������ѹǿ �ܼ�Сѹǿ�ݼ�С������B��Ũ�� �����ӷ�Ӧ���Ũ��
ƽ���ƶ��ķ�������Ӧ�����ƶ� ���淴Ӧ�����ƶ� �۲��ƶ�
| ��� | ƽ���ƶ������أ�����ţ� | ƽ���ƶ��ķ�������ţ� |
| A | ||
| B | ||
| C | ||
| D |
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �٢ۢ� |
| A�� | ��ʳ�����ůˮƿ�е�ˮ�� | |
| B�� | ��ά���������ڿ�ˮ��Ϊ�����ǣ��ʿ���Ϊ�����Ӫ������ | |
| C�� | ���ȵĴ���ˮϴ���� | |
| D�� | �������л������ڼ��Ի���������ɫ���ʿ����մ�۴�ۼ���ٺ�� |
 ��ϳɸø߾���ĵ������ȷ����ǣ�������
��ϳɸø߾���ĵ������ȷ����ǣ����������ڱ������ᡡ
�ڱ�ϩ��
�۱�ϩ����
���ڱ����״���
�ݱ�ϩ�ᣮ
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �٢� |
��pH=11�İ�ˮbL
�����ʵ���Ũ��Ϊ1��10-1mol•L-1�İ�ˮcL
��c��OH��=1��10-3mol•L-1��Ba��OH��2��ҺdL��
���ж�a��b��c��d��С��ϵ��ȷ��Ϊ��������
| A�� | c��a=d��b | B�� | b��a=d��c | C�� | c��a��b��d | D�� | a=b��c��d |

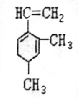 ��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������
��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������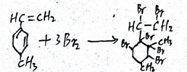 ��
��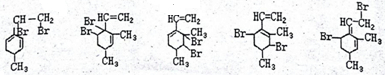 ��
��