��Ŀ����
9����ʽ̼������[NaaAlb��OH��c��CO3��d]��������ȼ����������ȣ����Ʊ������ǣ������¶ȡ�pH����NaHCO3ϡ��Һ�м���Al��OH��3�������裬��ַ�Ӧ����ˡ�ϴ�ӡ�����ü�ʽ̼����������1����ʽ̼������[NaaAlb��OH��c��CO3��d]��a��b��c��d֮��Ĺ�ϵΪa+3b=c+2d��
��2����ʽ̼��������Ϊ��ȼ���Ŀ���ԭ���ڷֽ�����д������ȣ��ڱ������������Ҳ���ȼ���۲�����ȼ������CO2��H2O��
��3����pH���ߣ���Բ�Ʒ��Ӱ����pH����ʹ��ʽ̼������ת��ΪNaAlO2��
��4��Ϊȷ����ʽ̼����������ɣ���������ʵ�飺
��ȷ��ȡ2.880g��Ʒ������ϡ�����ܽ⣬�õ�CO2 0.448L���ѻ���ɱ�״���£����������Һ�к���0.02mol Al3+��
�ڼ�����340������ʱ��ƷѸ�ٷֽ⣬�õ����������CO2��H2O������Ʒ�ֽ���ȫʱ����Ʒ�Ĺ�������ʣ�$\frac{������Ʒ��ʣ������}{������Ʒ����ʼ����}$��100%��Ϊ56.9%����������ʵ������ȷ����ʽ̼����������ɣ�д��������̣���
���� ��1����ʽ̼������[NaaAlb��OH��c��CO3��d]�У����ϼ۴�����Ϊ0��
��2����ʽ̼��������Ϊ��ȼ���Ŀ���ԭ���ڷֽ�����д������ȣ��ڱ������������Ҳ���ȼ����ͬ������֧��ȼ�յĶ�����̼��ˮ��
��3��pH���ߣ�����ǿ����������������������ƫ�����Σ�
��4����Ϊ��n��CO2��=$\frac{0.448L}{22.4L/mol}$��n��CO2��=0.02mol������n��H2O��=$\frac{2.880g����1-56.9%��-0.02mol��44g/mol}{18g/mol}$���������Һ�к���0.02molAl3+������b��c��d=1��2��1�����ݵ���غ�a+0.02��3=0.02��2+0.02��2������a=0.02mol������a��b��c��d=1��1��2��1���ɴ˷������
��� �⣺��1����ʽ̼������[NaaAlb��OH��c��CO3��d]�У����ϼ۴�����Ϊ0������a+3b-c-2d=0����a+3b=c+2d���ʴ�Ϊ��a+3b=c+2d��
��2����ʽ̼��������Ϊ��ȼ���Ŀ���ԭ���ڷֽ�����д������ȣ��ڱ������������Ҳ���ȼ����ͬ������֧��ȼ�յĶ�����̼��ˮ��
�ʴ�Ϊ��������ȼ������CO2��H2O��
��3��pH���ߣ�����ǿ����������������������ƫ�����Σ�����pH���ߣ���Բ�Ʒ��Ӱ���ǻ�ʹ��ʽ̼������ת��ΪNaAlO2��
�ʴ�Ϊ��pH����ʹ��ʽ̼������ת��ΪNaAlO2��
��4����Ϊ��n��CO2��=$\frac{0.448L}{22.4L/mol}$��n��CO2��=0.02mol������n��H2O��=$\frac{2.880g����1-56.9%��-0.02mol��44g/mol}{18g/mol}$���������Һ�к���0.02molAl3+������b��c��d=1��2��1�����ݵ���غ�a+0.02��3=0.02��2+0.02��2������a=0.02mol������a��b��c��d=1��1��2��1�����ԣ���ʽ̼�����Ļ�ѧ���ΪNaAl��OH��2CO3��
����Ϊ��n��CO2��=$\frac{0.448L}{22.4L/mol}$=0.02 mol����1�֣� n��CO2��=0.02 mol
���ԣ�n��H2O��=$\frac{2.880g����1-56.9%��-0.02mol��44g/mol}{18g/mol}$=0.02 mol
b��c��d=1��2��1�����ݵ���غ㣬a��b��c��d=1��1��2��1��
���ԣ���ʽ̼�����Ļ�ѧ���ΪNaAl��OH��2CO3 ��
���� ���⿼�黯ѧ����ʽ�йؼ��㣬Ϊ��Ƶ���㣬��ȷ����������֮���ϵ�ǽⱾ��ؼ���ע��ԭ���غ��������ã���Ŀ�Ѷ��еȣ�

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�| A�� | �������ƺ������Ƿֱ��ܽ���ˮ�� | B�� | �ɱ����Ȼ�立ֱ����ȱ�Ϊ���� | ||
| C�� | ʳ�κͱ��ֱ������ۻ� | D�� | Һ��;ƾ��ֱ�ӷ� |
| A�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� | |
| B�� | �ڹ��ۻ�������һ�����й��ۼ� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | �Ǽ��Լ�Ҳ�ɴ��������ӻ������� |
| ѡ�� | Ŀ�� | ���� |
| A | ����100mL1.0mol•L-1CuSO4 ��Һ | ��25.0gCuSO4•5H2O��������ˮ���100mL��Һ |
| B | ��ȥKNO3����������NaCl | ��������Ƴ��ȵı�����Һ����ȴ�ᾧ������ |
| C | ������Һ�Ƿ���SO42- | ȡ��������Һ���Թ��У����������ữ��Ba��NO3��2��Һ |
| D | ������Һ���Ƿ���NH4+ | ȡ������Һ���Թ��У�����NaOH���ȣ����Թܿڷ���һƬʪ��ĺ�ɫʯ����ֽ |
| A�� | A | B�� | B | C�� | C | D�� | D |
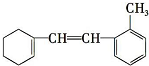
| A�� | �����ʱ����ϵ����ȴ����� 4 �� | |
| B�� | 1 mol �����ʺ� H2�ӳ������ҪH2�����ʵ���Ϊ 2 mol | |
| C�� | ��ʹ��ˮ��ɫ��1 mol �����ʺ���ˮ��ϣ�������� Br2�����ʵ���Ϊ 5 mol | |
| D�� | ������������ˮ����ʹ���Ը��������Һ��ɫ���ҷ������Ǽӳɷ�Ӧ |
| A�� | ����̫���ܡ���ϫ�ܡ��������磬���Ի�ȡ�����Դ | |
| B�� | ʳ���ͺ����Ͷ��������࣬����������ҵ���Ʒ��� | |
| C�� | ���ÿɽ���ġ��������ϡ�����һ���Է��У��ɷ�ֹ��ɫ��Ⱦ | |
| D�� | �ع�������������������������������˺�����Ӧ�����ӹ���������������� |
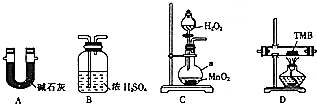 ��̼���⡢������Ԫ����ɵ�TMB��һ������ָ�Ƽ���ɫԭ�Լ�������Է�������Ϊ240��ij�о���ѧϰС���ͬѧ����������װ�òⶨTMB�ķ���ʽ��ʵ��ԭ�����������������н�4.80gTMB��Ʒ��������Ԫ��ת��ΪN2�������������ռ��ֱ�����ˮ������CO2�����ͼ��ѡ���ʵ���װ�ã�����װ�ÿ����ظ�������ʵ�飮
��̼���⡢������Ԫ����ɵ�TMB��һ������ָ�Ƽ���ɫԭ�Լ�������Է�������Ϊ240��ij�о���ѧϰС���ͬѧ����������װ�òⶨTMB�ķ���ʽ��ʵ��ԭ�����������������н�4.80gTMB��Ʒ��������Ԫ��ת��ΪN2�������������ռ��ֱ�����ˮ������CO2�����ͼ��ѡ���ʵ���װ�ã�����װ�ÿ����ظ�������ʵ�飮