��Ŀ����
11���л���A��C10H20O2������������ζ��������������ϴ���㲨�ķ��㸳�������֪��
��B������û��֧����
��D����̼��������Һ��Ӧ�ų�������̼��
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壮E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣�
��F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ�Т٢ڢܣ�ѡ����ţ���
��ȡ����Ӧ ����ȥ��Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��D��F���������Ĺ����ŵ������������Ȼ���̼̼˫����
��3��д����D��E������ͬ�����ŵ�ͬ���칹��Ŀ��ܽṹ��ʽ����CH3��2CHCH2COOH��CH3CH2CH��CH3��COOH��
��4��E����������������ù�صȣ���֪E���Ʊ�������ͬ���䳣����ͬϵ��ݱ���������2-��-1-�����ͼ�����һ����������ȡE���÷�Ӧ�Ļ�ѧ����ʽ�ǣ���CH3��2CHCH2OH+HCOOH$\stackrel{һ��������}{��}$��CH3��3CCOOH+H2O��
��5��ijѧ������C�Ĺ�����ʱ��ȡ1mol/LCuSO4��Һ��2mol/LNaOH��Һ��1mL����һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL40%��C�����Ⱥ���ɫ�������֣���ͬѧʵ��ʧ�ܵ�ԭ������Ǣۣ���ѡ����ţ�
�ټ����C���� �ڼ����C̫�� �ۼ���CuSO4��Һ�������� �ܼ���CuSO4��Һ����������
���� B������������D��D����̼��������Һ��Ӧ�ų�������̼��D�����Ȼ�����BΪ����CΪȩ��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壬D��EΪ���ᣬ��AΪ������B��C��D��E��F������̼ԭ������ͬ��A�ķ���ʽΪC10H20O2�����ڱ���һԪ������B�ķ���ʽΪC5H12O��E�ķ���ʽΪC5H10O2��B��������֧������B�ṹ��ʽΪCH3��CH2��3CH2OH����CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣���E�Ľṹ��ʽΪ��CH3��3CCOOH��B��E����������Ӧ����A����AΪC��CH3��3COOCH2��CH2��3CH3��F����ʹ������Ȼ�̼��Һ��ɫ����B��Ũ���ᡢ���������·�����ȥ��Ӧ����F����F�Ľṹ��ʽΪCH3��CH2��2CH=CH2���ݴ˽��н��
��� �⣺B������������D��D����̼��������Һ��Ӧ�ų�������̼��D�����Ȼ�����BΪ����CΪȩ��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壬D��EΪ���ᣬ��AΪ������B��C��D��E��F������̼ԭ������ͬ��A�ķ���ʽΪC10H20O2�����ڱ���һԪ������B�ķ���ʽΪC5H12O��E�ķ���ʽΪC5H10O2��B��������֧������B�ṹ��ʽΪCH3��CH2��3CH2OH����CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣���E�Ľṹ��ʽΪ��CH3��3CCOOH��B��E����������Ӧ����A����AΪC��CH3��3COOCH2��CH2��3CH3��F����ʹ������Ȼ�̼��Һ��ɫ����B��Ũ���ᡢ���������·�����ȥ��Ӧ����F����F�Ľṹ��ʽΪCH3��CH2��2CH=CH2��
��1��BΪCH3��CH2��3CH2OH�����еĹ�����Ϊ�ǻ������Է���ȡ����Ӧ������ȼ�գ�����������Ӧ�����Է������������ܷ�����ȥ��Ӧ�����ܷ����Ӿ۷�Ӧ��
�ʴ�Ϊ���٢ڢܣ�
��2��DΪCH3��CH2��3COOH�����й�����Ϊ�Ȼ����ṹ��ʽΪ-CHO��FΪCH3��CH2��2CH=CH2�����������Ĺ�������̼̼˫����
�ʴ�Ϊ���Ȼ���̼̼˫����
��3����D��E������ͬ�����ŵ����ʽṹ��ʽ����CH3��2CHCH2COOH��CH3CH2CH��CH3��COOH��
�ʴ�Ϊ����CH3��2CHCH2COOH��CH3CH2CH��CH3��COOH��
��4����2һ��-1-�����ͼ�����һ����������ȡE���÷�Ӧ�Ļ�ѧ����ʽ�ǣ���CH3��2CHCH2OH+HCOOH$\stackrel{һ��������}{��}$��CH3��3CCOOH+H2O��
�ʴ�Ϊ����CH3��2CHCH2OH+HCOOH$\stackrel{һ��������}{��}$��CH3��3CCOOH+H2O��
��5��CΪCH3��CH2��3CHO������ȩ���������Ƶ�������ͭ���飬��Ҫ�ټ��������¡����ȣ�ȡ1mol/LCuSO4��Һ��2mol/LNaOH��Һ��1mL������ǡ�÷�Ӧ����������ͭ�����������ͭ���࣬���Ǽ����������ʼ�����ɫ�������֣�
�ʴ�Ϊ���ۣ�
���� ���⿼���л�����ƶϣ���Ŀ�ѶȽϴ��漰����ȩ�������������ת���ȣ��Ѷ��еȣ�������Ŀ��Ϣ����Ϸ�Ӧ������A�ķ���ʽ�����ƶϣ�ȷ��B��C��D��E��F������̼ԭ������ͬ�ǽ���Ĺؼ���

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�| A�� | �����ȼ����Ϊ890.3kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3kJ•mol-1 | |
| B�� | ��֪ǿ����ǿ����ϡ��Һ�ﷴӦ���к���Ϊ57.3 kJ•mol-1���� $\frac{1}{2}$H2SO4��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{2}$BaSO4��s��+H2O��l����H=-57.3 kJ•mol-1 | |
| C�� | 500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��$?_{500��/30MPa}^{����}$ 2NH3��g����H=-38.6kJ•mol-1 | |
| D�� | ��֪25�桢101KPa�����£�4Al��s��+3O2��g���T2A12O3��s����H=-2834.9 kJ•mol-1��4Al��s��+2O3��g���T2A12O3��s����H=-3119.1 kJ•mol-1����O2��O3�ȶ� |
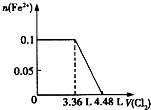 ��1�����е����ʵ�����SO32-��Fe2+��Br-��I-����Һ�У�ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ�����ϵ��ͼ��ʾ��
��1�����е����ʵ�����SO32-��Fe2+��Br-��I-����Һ�У�ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ�����ϵ��ͼ��ʾ����ԭ��Һ�и����ӵ����ʵ�����0.1mol
��ͨ��C123.36Lʱ����Һ�з�����Ӧ�����ӷ���ʽ��SO32-+Cl2+H2O=SO42-+2Cl-+2H+��2I-+Cl2=I2+2Cl-��
��2��ij��Һ�н�������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩���Ҹ����ӵ����ʵ�����Ϊ0.01mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
b������Һ�м�����������ᣬ���������ɣ���Һ�������������
c������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
�Իش��������⣺
��ԭ��Һ����������������NO3-��Cl-��SO42-��
������ԭ��Һ�м�������NaOH��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù�����CuO��Fe2O3��д��ѧʽ����
������ԭ��Һ�м���������ᣬд���ù����з�����Ӧ�����ӷ���ʽ3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
 �����й���PHB�������в���ȷ���ǣ�������
�����й���PHB�������в���ȷ���ǣ�������| A�� | ���ܷ���ˮ�ⷴӦ | |
| B�� | �������������¿ɽ����CO2��ˮ | |
| C�� | ����������һ�ֺ��ǻ������ᾭ���۷�Ӧ���� | |
| D�� | �Ʊ����ĵ���ΪHCOOH��CH3CH2CH2OH |
| A�� | �� SO2ͨ��ˮ�� | B�� | �ռ�����ˮ | C�� | �� HCl ͨ��ˮ�� | D�� | NaHSO4����ˮ�� |
| A�� | SiO2��N2O5��CO��Cl2 | B�� | Al2O3��Cl2��N2O5��SO3 | ||
| C�� | CO2��Al��OH��3��CaO��SO2 | D�� | Al2O3��CO2��SO3��SO2 |

 ��
�� ��
�� �١�����Ԫ�����ڱ���ǰ�����ڵ�8��Ԫ�أ������λ����ͼ��ʾ��
�١�����Ԫ�����ڱ���ǰ�����ڵ�8��Ԫ�أ������λ����ͼ��ʾ�� ��
��