��Ŀ����
1��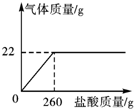 �ҹ��̲��ŷḻ�ĺ�����Դ������ˮ��Դ��ȱ����Ҫ�������ã�����ˮ��Դ��
�ҹ��̲��ŷḻ�ĺ�����Դ������ˮ��Դ��ȱ����Ҫ�������ã�����ˮ��Դ����1���ø߷��Ӳ�������ķ���Ĥ���Խ���ˮ��Ϊ��ˮ���˹�����Ҫ������������ѡ���������ѧ�����仯��
��2������þ���ԴӺ�ˮ�����Ȼ�þ������ȡ���������ͼ��ʾ��
��ˮ��±ˮ$\stackrel{��ʯ��}{��}$������þ$\stackrel{����}{��}$�Ȼ�þ$\stackrel{ͨ��}{��}$þ
�ش��������⣺
������������û���漰�Ļ�����Ӧ�������û���
��д������ת����������Ԫ�ػ��ϼ������Ļ�ѧ����ʽMgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2����
��3���Ӻ�ˮ����ȡ�Ĵ����к���������ɳ������þ���Ȼ��Ƶ����ʣ�Ϊ�õ��ϴ����Ȼ��ƣ�����������ˮ��Ȼ��������²�����a���ӹ�����Na2CO3 ��Һ��b�������������c��������d�����ˣ�e�ӹ�����Ba��OH��2��Һ����ȷ�IJ���˳����eadbc������ĸ���ţ�����д������Ba��OH��2��Һ������Ӧ�Ļ�ѧ����ʽBa��OH��2+MgSO4=BaSO4��+Mg��OH��2����
��NH3+CO2+H2O�TNH4HCO3��
��NH4HCO3+X=NH4Cl+NaHCO3��
��.2NaHCO3 $\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2��
�����й�������X����������Ϊ�����ӡ������ӣ���ȥ����Na2CO3��ĩ������NaHCO3�ķ����Ǽ��ȣ�
��ȡ55gij��ҵ��������м�������ϡ���ᣨ�����ʲ���Ӧ��������������������������������Ĺ�ϵ��ͼ��ʾ����˹��մ�����̼���Ƶ����������Ƕ��٣���������������0.1%����
���� ��1����ˮ�������̣�û�в����µ����ʣ����������仯�����Ծݴ˽����⣻
��2��̼��Ƹ����ֽܷ����������ƺͶ�����̼������������ˮ��Ӧ�����������ƣ��������������Ȼ�þ��Ӧ����������þ�������Ȼ��ƣ�������þ�������ᷴӦ�����Ȼ�þ��ˮ���Ȼ�þ��ͨ��ֽ�����þ��������
��3�����ݴ����е����ʣ��ҳ������Լ���˳��
��4���ٸ��ݻ�ѧ��Ӧǰ��ԭ���������Ŀ���䣬�ƶϷ�Ӧ��ij���ʵĻ�ѧʽ������Na2CO3��NaHCO3�����ʽ��
�ڸ��ݶ�����̼��������ϻ�ѧ����ʽ���̼���Ƶ��������������ҵ������̼���Ƶ�����������
��� �⣺��1����ˮ�������̣�û�в����µ����ʣ����������仯��
��2��������Ӧ�У�̼��Ʒֽ����������ƺͶ�����̼���ǷֽⷴӦ���������������Ȼ�þ��Ӧ����������þ�������Ȼ��ƣ��Ǹ��ֽⷴӦ��������������ȼ������ˮ���ǻ��Ϸ�Ӧ��û���漰���û���Ӧ����Ӧ���л��ϼ۱仯�ķ�Ӧ��MgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2����
����û���MgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2��
��3��Ҫ��ȥ�����к��е����ʣ����Լӹ���������������Һ��ȥ����þ���ӹ�����Na2CO3��Һ��ȥ�Ȼ��ƺ����Լ�����������ͨ�����˰Ѳ�����ˮ�����ʳ�ȥ���������������ȥ������̼���ƣ�ͨ��������ȥ���������������Һ��ȥ����þ���仯ѧ����ʽΪBa��OH��2+MgSO4=BaSO4��+Mg��OH��2����
��4���ٸ��ݻ�ѧ��Ӧǰ��ԭ���������Ŀ���䣬�ƶϷ�Ӧ��ij���ʵĻ�ѧʽ��NaCl��Na2CO3�ȶ���NaHCO3�����ֽ⣬��ȥ����Na2CO3��ĩ������NaHCO3���ü��ȵķ�����
����������������� 22g����˹�ҵ������̼���Ƶ�����Ϊ x��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 22g
$\frac{106}{x}=\frac{44}{22g}$��x=53g
�ʴ˹�ҵ������̼���Ƶ����������ǣ�$\frac{53g}{55g}$��100%=96.4%��
�𣺴˹�ҵ������̼���Ƶ����������� 96.4%��
�ʴ�Ϊ��
��1������
��2�����û��� ��MgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2��
��3��eadbc�� Ba��OH��2+MgSO4=BaSO4��+Mg��OH��2����
��4���������ӡ������ӣ�����
��96.4%
���� ���⿼�������غ㶨�ɺ���ȡ������Ϣ�ó��������ʲ�������г��ӣ������˷���ʽ�ļ��㣬ע������Ӧ����Һ��������������ʱ��Ҫע����Ʒ�е������Ƿ�����Һ�����ʵ�һ���֣�

| A�� | �����������ڻ�ѧ��Ӧǰ��һ������ | |
| B�� | �κη�Ӧ�еĶ�������һ���Ǵ��� | |
| C�� | �������ܸı�������IJ��� | |
| D�� | �����ڻ�ѧ��Ӧ��ܼӿ췴Ӧ���ʣ����������������ʾ����� |
| A�� | ����K+ | B�� | ������H2O | C�� | ������OH- | D�� | һ����OH- |
| A�� | ������ˮ�Ƴɱ��� | |
| B�� | ��ʳ�׳�ȥůˮƿ�е�ˮ�� | |
| C�� | ����ʱ���봿���Գ�ȥ���������ζ | |
| D�� | �й����ߵIJ˵�δ������������ |
 С����װ����ͼ��ʾ��ʵ��װ�ã�С�Թ���װ�е����ķ�״̼��ƺͿ�״̼��ƣ���ͷ�ι���װ����ͬŨ�Ⱥ������ϡ���ᣬʵ�鿪ʼʱ�����ι��е�ϡ����ͬʱ���뵽С�Թ��У��۲�ʵ������
С����װ����ͼ��ʾ��ʵ��װ�ã�С�Թ���װ�е����ķ�״̼��ƺͿ�״̼��ƣ���ͷ�ι���װ����ͬŨ�Ⱥ������ϡ���ᣬʵ�鿪ʼʱ�����ι��е�ϡ����ͬʱ���뵽С�Թ��У��۲�ʵ������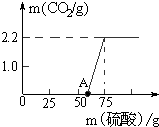 ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����