��Ŀ����
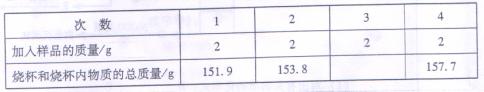
ij���������к�̼������ʡ���ȡ��ϸ�ĸ���Ʒ12.4g������ƿ�У�����32.6g��ˮ��������γ�����Һ��������ƿ����εμ�����ʹ���ַ�Ӧ�������ݲ�������ü����������������ƿ�е����ʵ�������ϵ���±�ʾ��
| �������������g | 0 | 25 | 37.5 |
| ��ƿ�����ʵ����� | 45 | 70 | 80.3 |
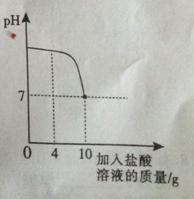
��1�����������������0—25gʱ�� �����ᷢ����Ӧ��
��2����Ӧ����������̼����Ϊ g��
��3������Ʒ���������Ƶ�����������д��������̣���ȷ��0.1%����
���𰸡���1���������ƣ�2��2.2g
��3���⣺�����Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 2.2g
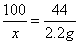
X=5g
����Ʒ���������Ƶ���������Ϊ��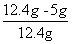 ��100%��59.7%
��100%��59.7%
����Ʒ���������Ƶ���������Ϊ59.7%��
���������ɱ������ݷ�����֪���������������Ϊ0——25ʱ�����������������ᷢ���кͷ�Ӧ��������37.5g����ʱ�����Ķ�����̼������Ϊ��37.5g+12.4g+32.6g-80.3g=2.2g�����ݻ�ѧ����ʽ���Լ������Ʒ��̼��Ƶ�������������Ʒ����������������Ƶ�����������

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�Ϊ�˲ⶨij������Ʒ(�������Ȼ�������)��̼���Ƶ�����������ȡ����Ʒ������ϡ�������ձ��з�Ӧ���й�ʵ���������±�
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ������ | �ձ���ϡ�����������g | ������Ʒ��������g | �ձ������л�����������g |
| 120 | 12 | 127��6 |
����㣺
(1)��Ӧ���ɶ�����̼������Ϊ g��
(2)�ô�����Ʒ��̼���Ƶ���������Ϊ���١�(��д���������)
��ǰ���в�����Сѧ���С�����ʳ�á����̡��±�ΪijУʳ��ij����Ͳ���ʳ�ס�
| ��ʳ | ��� | �߲� |
| �� | ����ţ�� | �����ܲ������ƹ� |
(1)ʳ���и��������ʵ���________������ά���ص���________(���ϱ��е�һ����ʳ�����)��������Ҫ������������Ӫ������______________��
(2)ʳ����ù��Ĵ���________��
A.����ʳ�á�B.��������ʳ�á�C.���Բ���ʳ��
(3)ͬѧ��ѧУʳ�õ����н��鲻��������________��
A.����ʳ�в���������� B.���ṩ��ըʳ��
C.�ʵ��ṩˮ��
 ��
��