��Ŀ����
8���ҹ�������ѧ�Һ�°����ġ������Ƽ������ԭ������ʳ��ˮ��ͨ���������������ͣ�Ȼ���ټ�ѹ������ͨ�����������̼����������NaHCO3���壬�ٽ�����ȼ��õ������Ӧ�Ļ�ѧ����ʽΪ��NaCl+H2O+CO2+NH3�TNaHCO3+NH4Cl
2NaHCO3 $\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O
��1��ֻ��NaCl��CO2���Ƶ�NaHCO3��ԭ����NaCl��CO2�ж�������Ԫ�أ�
��2����ҵ��ȡCO2�Ļ�ѧ����ʽ��CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2����
��3�����������̿�֪����Na2HCO3�����л�������NaHCO3���ʣ����ü��ȷ�����NaHCO3��ȥ��
��4�����������쵪����ԭ��֮һ��ʵ������п��ϡ���ᷴӦ��ȡ�������۱�����пԭ���������ӷ�Ӧ����п���Ӻ�����ӣ�
���� ��1�����������غ㶨�����������
��2����ҵ��ͨ���ø�������ʯ��ʯ�ķ�������ȡ������̼��
��3���������ʵ����ȶ�����������
��4���ӻ�ѧ�仯��ʵ����������
��� �⣺��1�����ݻ�ѧ�仯ǰ���Ԫ���غ��֪��NaCl��CO2�в�����Ԫ�أ��������Ƶ�NaHCO3�����NaCl��CO2�ж�������Ԫ�أ�
��2��ʯ��ʯ����Ҫ�ɷ���̼��ƣ��ڸ��µ������·ֽ�Ϊ�����ƺͶ�����̼�����CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2����
��3��̼�������Ȳ��ֽ⣬��̼�������ڼ��ȵ������·ֽ�Ϊ̼���ơ�ˮ�Ͷ�����̼�����Կ��ü��ȵķ�����ȥ̼�����л��е�̼�����ƣ�������ȣ�
��4��п��ϡ���ᷴӦ��������п���������䷴Ӧ���۱�����пԭ���������ӷ�Ӧ����п���Ӻ�����ӣ����пԭ���������ӷ�Ӧ����п���Ӻ�����ӣ�
���� ʵ������������֮������õ����ڱ��֣����Ҫѧ�����ʵ�顢�۲�ʵ�顢����ʵ�飬Ϊ��ʾ����֮������õ�ʵ�ʵ춨������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
19����ȤС��ͬѧ��ʵ���Ҵ�����Һʱ����һƿ���н�����ʯ��ȡ�������뺬������ķ�Һ�У����ַ�Һ�в������������ݣ����ǽ�һ���˽���ʯ����Ʒ���ʵij̶Ƚ�������̽�����
��̽���һ�������ʯ����Ʒ�Ƿ���ȫ����
��̽��������ⶨ���ʵ���ʯ����Ʒ��̼��Ƶ���������������ͼ��ʾװ�ú��Լ�����ʵ�飨����̨��ȥ��װ�����������ã���������Ļӷ�����ÿ����Ӧ�����ö�����ȫ�ģ�����ȡ5g����Ʒ��ͨ���ⶨ��Ʒ��ϡ����
��Ӧ���������������������̼��Ƶ�����������
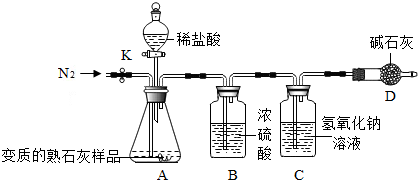
��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
����ͼ����װ�ã���C��D�⣩������5.00g����Ʒ������ʱʹ�õ�����ƽ����
�۳�������¼װ��C��������Ϊ200.00g������ʱע����C�����ˣ���
�ܴ��ɼ�K������N2Լ1���ӣ�
�ݽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����ܲ�����������װ��C������Ϊ200.88g������ʱע����C�����ˣ���
���㣮
�ش�����������⣺
��1��д��A�в�������ķ�Ӧ��ѧ����ʽCaCO3+2HCl�TCaCl2+H2O+CO2����
��2��װ��B��Ũ���������������ˮ�֣���֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������Ƿ�ֹ�����е�CO2����Cװ���У�
��3��������м���ϡ���ᣬ�����ٲ�������Ϊֹ����Ŀ����ʹ��CaCO3��ȫ��Ӧ��
��4���������װ��C�������������ӣ�˵��CO2��NaOH��ȫ���գ�д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH�TNa2CO3+H2O��
��5��������ɵ�CO2���������Ϊ0.88gg����Ʒ��̼��Ƶ�����Ϊ2gg���ⶨ���ʵ���ʯ����Ʒ��̼��Ƶ���������=40%��
��6�����в����ᵼ�²������ƫ�͵���D������ţ���
A��ʡ��װ��B B��û�н��в���ܵIJ���C������N2ʱ��ϳ� D�������û���ظ�����ܵIJ�����
��̽���һ�������ʯ����Ʒ�Ƿ���ȫ����
| ʵ����� | ʵ������ | ʵ����� |
| ȡ�����Թ��У���ˮ�ܽ⣬���ϲ���Һ�еμ���ɫ��̪ | ��Һ�Ժ�ɫ | ���ʵ���ʯ�����Ժ��������� |
��Ӧ���������������������̼��Ƶ�����������

��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
����ͼ����װ�ã���C��D�⣩������5.00g����Ʒ������ʱʹ�õ�����ƽ����
�۳�������¼װ��C��������Ϊ200.00g������ʱע����C�����ˣ���
�ܴ��ɼ�K������N2Լ1���ӣ�
�ݽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����ܲ�����������װ��C������Ϊ200.88g������ʱע����C�����ˣ���
���㣮
�ش�����������⣺
��1��д��A�в�������ķ�Ӧ��ѧ����ʽCaCO3+2HCl�TCaCl2+H2O+CO2����
��2��װ��B��Ũ���������������ˮ�֣���֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������Ƿ�ֹ�����е�CO2����Cװ���У�
��3��������м���ϡ���ᣬ�����ٲ�������Ϊֹ����Ŀ����ʹ��CaCO3��ȫ��Ӧ��
��4���������װ��C�������������ӣ�˵��CO2��NaOH��ȫ���գ�д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH�TNa2CO3+H2O��
��5��������ɵ�CO2���������Ϊ0.88gg����Ʒ��̼��Ƶ�����Ϊ2gg���ⶨ���ʵ���ʯ����Ʒ��̼��Ƶ���������=40%��
��6�����в����ᵼ�²������ƫ�͵���D������ţ���
A��ʡ��װ��B B��û�н��в���ܵIJ���C������N2ʱ��ϳ� D�������û���ظ�����ܵIJ�����
16������˵������ȷ���ǣ�������
| A�� | ��ʯ�ҿ����������������Լ��к��������� | |
| B�� | ��ˮ����ԭ���ǽ����˿�ȼ����Ż�� | |
| C�� | ��C��H2��CO�����Խ�CuO��ԭ��Cu | |
| D�� | ȼ�պͻ����������Ƿ��ȷ�Ӧ |
3��ʵ�������ù������⡢����ء�������صȶ�������ȡ��������ԭ���ǣ�������
| A�� | �������� | B�� | ���������� | C�� | ��������Ԫ�� | D�� | �������������� |
13���淶�IJ�����ʵ��ɹ��Ͱ�ȫ�ı�֤������ʵ�������ȷ���ǣ�������
| A�� |  CO2���� | B�� |  ��ȼ�ƾ��� | C�� |  �μ�Һ�� | D�� |  ϡ��Ũ���� ϡ��Ũ���� |



 ������ˮ�ķֲ��ǣ�������ˮ97%����ˮ3%������ˮ�ķֲ�����ͼ��ʾ���ش�
������ˮ�ķֲ��ǣ�������ˮ97%����ˮ3%������ˮ�ķֲ�����ͼ��ʾ���ش�