��Ŀ����
18�� ������ˮ�ķֲ��ǣ�������ˮ97%����ˮ3%������ˮ�ķֲ�����ͼ��ʾ���ش�
������ˮ�ķֲ��ǣ�������ˮ97%����ˮ3%������ˮ�ķֲ�����ͼ��ʾ���ش���1��δ����ȡ������ˮ��Դ��������Ҫ;����AC����ѡ���
A����ˮ���� B������ˮ��������
C������ȡˮ D����ȿ�������ˮ
��2�������ͺ�����ˮ��Դ��Ȼ���ޣ�����������ˮ��Ҫ�ɼ��ڴˣ�һ����������ˮҪ�������������ˡ������ȹ��̣�
�١�����������ˮ�м������������е���������ˮ��Ӧ�����������Ե�������������ɻ�ѧ��Ӧʽ����ƽ��Al3++3H2O�TAl��OH��3��+3H+
�ڡ�����������Cl2��������������ˮ��Ӧ���ɵ�HClO�������ᣩ���������ã�����Ư���г����д�����ƣ��仯ѧʽΪCa��ClO��2��
���� ��1������δ����ˮ��Դ����Դ��������
��2���ٸ��ݷ�Ӧǰ��ԭ�ӵ��������Ŀ���������ı���������
�ڸ��ݻ�����Ļ�ѧʽ��д����������
��� �⣺��1��A����ˮ�к��д�����ˮ�֣����ú�ˮ���εķ�������ȡ��ˮ��
B�������к��е�ˮ�������٣��˷�����ʵ�ʣ�
C�������̲��ŷḻ��ˮ��Դ�������ô˷�����ȡ��ˮ��
D����ȿ�������ˮ�������Ǻõķ�����
���AC��
��2���ٸ��������غ㶨���Լ���ɵ��غ��֪���÷�ӦʽΪ��Al3++3H2O�TAl��OH��3��+3H+�����3��3��
�ڸ�Ԫ����+2�ۣ����������-1�ۣ��ʴ�����ƵĻ�ѧʽΪ��Ca��ClO��2�����Ca��ClO��2��
���� ���⿼���˵�ˮ����Դ����ѧ��Ӧʽ����ƽ�Լ�������Ļ�ѧʽ��д�����ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
6����֪��ѧ��ӦAl2O3+2NaOH=2R+H2O���ƶ�R�Ļ�ѧʽ�ǣ�������
| A�� | Na2O | B�� | Al��OH��3 | C�� | NaAlO2 | D�� | NaAlO |
13��ҽ�����鸹к������ʱ��ʳ�ø��������ʺ���֬��ʳ�����ѡ�������ǣ�������
| A�� | �����Ͷ��� | B�� | ������ţ�� | C�� | ��ͷ��ϡ�� | D�� | ��������� |
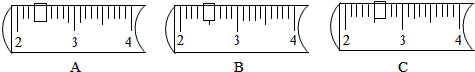
4������ʵ������У�����ȷ���� ��������
| A�� | ����ʱ��������ȡ������ | |
| B�� | ֹͣ����ʱ���õ�ñ����ƾ��� | |
| C�� | ���Խ���ʴ�Ե�ҩƷֱ�ӷ��������ϳ��� | |
| D�� | ����ʱ��ˣ��Һ���ز�����������©�����㵹�㵹 |