��Ŀ����
19����ȤС��ͬѧ��ʵ���Ҵ�����Һʱ����һƿ���н�����ʯ��ȡ�������뺬������ķ�Һ�У����ַ�Һ�в������������ݣ����ǽ�һ���˽���ʯ����Ʒ���ʵij̶Ƚ�������̽�������̽���һ�������ʯ����Ʒ�Ƿ���ȫ����
| ʵ����� | ʵ������ | ʵ����� |
| ȡ�����Թ��У���ˮ�ܽ⣬���ϲ���Һ�еμ���ɫ��̪ | ��Һ�Ժ�ɫ | ���ʵ���ʯ�����Ժ��������� |
��Ӧ���������������������̼��Ƶ�����������
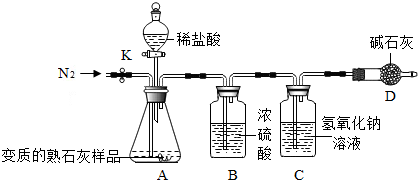
��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
����ͼ����װ�ã���C��D�⣩������5.00g����Ʒ������ʱʹ�õ�����ƽ����
�۳�������¼װ��C��������Ϊ200.00g������ʱע����C�����ˣ���
�ܴ��ɼ�K������N2Լ1���ӣ�
�ݽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����ܲ�����������װ��C������Ϊ200.88g������ʱע����C�����ˣ���
���㣮
�ش�����������⣺
��1��д��A�в�������ķ�Ӧ��ѧ����ʽCaCO3+2HCl�TCaCl2+H2O+CO2����
��2��װ��B��Ũ���������������ˮ�֣���֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������Ƿ�ֹ�����е�CO2����Cװ���У�
��3��������м���ϡ���ᣬ�����ٲ�������Ϊֹ����Ŀ����ʹ��CaCO3��ȫ��Ӧ��
��4���������װ��C�������������ӣ�˵��CO2��NaOH��ȫ���գ�д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH�TNa2CO3+H2O��
��5��������ɵ�CO2���������Ϊ0.88gg����Ʒ��̼��Ƶ�����Ϊ2gg���ⶨ���ʵ���ʯ����Ʒ��̼��Ƶ���������=40%��
��6�����в����ᵼ�²������ƫ�͵���D������ţ���
A��ʡ��װ��B B��û�н��в���ܵIJ���C������N2ʱ��ϳ� D�������û���ظ�����ܵIJ�����
���� ��̽���һ�������÷�̪��Һ�����Ƿ����������ƣ���ϡ��������Ƿ���̼��ƣ�Ȼ��������ó���ȷ�Ľ��ۣ�
��̽���������1������ϡ������̼��Ƶķ�Ӧд����Ӧ�ķ���ʽ��
��2������Ũ��������ˮ�ԣ��������������ն�����̼�����ش�
��3������ϡ������̼��Ƶķ�Ӧ��������
��4������������Һ�����ն�����̼������̼���ƺ�ˮ��
��5������������Һ�����ն�����̼��װ��C�������仯��Ϊ������̼�����������ݶ�����̼��������Ϸ���ʽ���㣮
��6��A��װ��B��ʹA�лӷ�����ˮ����һͬ����C��
B��û�н��в���ܵIJ�������ʹװ����ԭ�еĶ�����̼��C���գ�
C������N2ʱ��ϳ����Խ����Ӱ�죻
D�������û���ظ�����ܵIJ��������ɵĶ�����̼����ͣ����AB�У�
��� �⣺��̽���һ���������������ܹ��Ϳ����еĶ�����̼������Ӧ����̼��ƶ����±��ʣ�����ȫ������ֻ��̼��ƣ���û�б�����ֻ���������ƣ������ֱ�������̼��ƺ��������ƣ��������������Ǽ��̼�����̼���Σ�����������Һ��ʹ��̪��Һ��죬
��̽���������1����װ��A�У������ϡ��������̼��Ʒ�Ӧ����Ӧ�ķ���ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2����
��2������Ũ��������ˮ�ԣ�����װ��D�ļ�ʯ�ҿ������տ����ж�����̼���壬��ֹ�����е�CO2����Cװ���жԲⶨ����Ӱ�죻
��3������ϡ���ᣬ�����ٲ������ݣ�˵��CaCO3��ȫ��Ӧ��
��4������������Һ�����ն�����̼������̼���ƺ�ˮ��װ��C�������������ӣ�˵��CO2��NaOH��ȫ���գ���ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
��5��װ��C�������仯��Ϊ������̼��������200.88g-200.00g=0.88g��
����Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 0.88g
$\frac{100}{x}$=$\frac{44}{0.88g}$
x=2.00g
���ʵ���ʯ����Ʒ��̼��Ƶ���������$\frac{2.00g}{5.00g}$��100%=40%
��6��A��װ��B��ʹA�лӷ�����ˮ����һͬ����C��ʹ���ƫ��
B��û�н��в���ܵIJ�������ʹװ����ԭ�еĶ�����̼��C���գ�ʹ���ƫ��
C������N2ʱ��ϳ����Խ����Ӱ�죻
D�������û���ظ�����ܵIJ��������ɵĶ�����̼����ͣ����AB�У�û�б�C���գ�ʹ���ƫС��
�ʴ�Ϊ����̽���һ��
| ʵ����� | ʵ������ | ʵ����� |
| ȡ�����Թ��У���ˮ�ܽ⣬���ϲ���Һ�еμ���ɫ��̪ | ��Һ�Ժ�ɫ | ���ʵ���ʯ�����Ժ��������� |
��1��CaCO3+2HCl�TCaCl2+H2O+CO2��
��2������ˮ�֣���ֹ�����е�CO2����Cװ����
��3��ʹ��CaCO3��ȫ��Ӧ
��4��CO2��NaOH��ȫ����
CO2+2NaOH�TNa2CO3+H2O
��5��0.88g 2g 40%
��6��D
���� �����ۺϿ������������ƺ�̼��Ƶ����ʡ���д����ʽ�����������������й��������ƺ�̼��Ƶ�֪ʶ�ǽ����Ĺؼ���

 ������������ϵ�д�
������������ϵ�д�| A�� | �������� | |
| B�� | ��Ԫ�ص�������������ʽΪ$\frac{6}{176}$��100% | |
| C�� | ÿ�������к���20��ԭ�� | |
| D�� | ̼���⡢������Ԫ�ص�������3��4��3 |
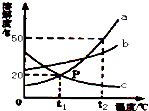 ��ͼ��a��b��c�������ʣ��������ᾧˮ�����ܽ�����ߣ��ش𣺣�1��P��ĺ�����t1��ʱa��c���ܽ����ȣ�
��ͼ��a��b��c�������ʣ��������ᾧˮ�����ܽ�����ߣ��ش𣺣�1��P��ĺ�����t1��ʱa��c���ܽ����ȣ� ij�����������Na2SO4��NaCl��Na2CO3��KNO3�е�һ�ֻ�����ɣ��ֽ�������ʵ�飮
ij�����������Na2SO4��NaCl��Na2CO3��KNO3�е�һ�ֻ�����ɣ��ֽ�������ʵ�飮