��Ŀ����
8��ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʣ��������Ʊ����ε�ʵ�鷽�������������������£�
��1����������ƽ��������ʱ����ָ��ƫ���ұߣ����ʾ����������ȷѡ��Ĵ��룩D��
A�������أ������� B�������ᣬ��Ʒ��
C�������أ���Ʒ�� D�������ᣬ������
��2���ڢڲ�������Ŀ���dz�ȥ�����е�Na2SO4���ѧʽ����ͬ�����ڢ�������Ŀ���dz�ȥ��Һ��NaOH��Na2CO3��
��3���ڢ۲�����������Ӧ�Ļ�ѧ����ʽ��2NaOH+MgCl2=Mg��OH��2��+2NaCl��
��4���ڢݲ������ˡ������еõ������ijɷ��У���ɳ��BaSO4��Mg��OH��2��CaCO3��BaCO3���ѧʽ����
���� ��1�����ݳ��������ǡ��������롱���н��
��2�������е�MgCl2��CaCl2��Na2SO4������ˮ��Ҫ�뽫���ʳ�ȥ���뽫MgCl2��CaCl2��Na2SO4ת��Ϊ��������ͬ��ɳһ����˳�ȥ��
��� �⣺��1�����������ǡ��������롱��������������ƽ��������ʱ����ָ��ƫ���ұߣ����ʾ�����ᣬ�����أ�
��2���ڢڲ������������BaCl2��Һ����Na2SO4������Ӧ������BaSO4������NaCl���ڼ������BaCl2��Һ����Na2SO4������Ӧ������BaSO4������NaCl��
�ۼӹ���NaOH��Һ����MgCl2������Ӧ������Mg��OH��2�������Ȼ��ƣ���Ӧ�ķ���ʽΪ��2NaOH+MgCl2=Mg��OH��2��+2NaCl��
�ܼӹ���Na2CO3��Һ����CaCl2�͢��й���BaCl2��Һ������Ӧ������CaCO3������BaCO3�������Ȼ��ƣ�
�ݹ��ˣ��˳�ǰ�����ɵ�BaSO4��Mg��OH��2��CaCO3��BaCO3��������ɳ��
���������ᣬ����й���NaOH��Һ�͢��й���Na2CO3��Һ������Ӧ�������Ȼ��ơ�ˮ�Ͷ�����̼���壮
���������ᾧ���õ����Σ�
�ʴ�Ϊ����1��D�� ��2��Na2SO4 NaOH��Na2CO3��
��3��2NaOH+MgCl2=Mg��OH��2��+2NaCl�� ��4��BaCO3��
���� ������Ҫ����ѧ���Դ����ᴿʵ������������̶ȸ�������Һ����Ϊ���壬��������������������;�������IJ��衢����淶������������������仯����Ͳ�Ķ������������������ļ�������⣬���ʱҪ����ͼʾ����Ͼ���֪ʶ����н��������ѧ���ۺ���������ߣ����ڱ�������ǿ����ϸ�ڵ���Ҫ�ԣ�ϸ�ھ����ɰ�

 ��β����ת�������ǽ�����β���е��ж�����ת��Ϊ�����壮���۹�����ͼ��ʾ�����в�ͬ��Բ�������ͬ��ԭ�ӣ�����˵������ȷ���ǣ�������
��β����ת�������ǽ�����β���е��ж�����ת��Ϊ�����壮���۹�����ͼ��ʾ�����в�ͬ��Բ�������ͬ��ԭ�ӣ�����˵������ȷ���ǣ�������| A�� | ��Ӧ���������ȫ���ǻ����� | B�� | ������ķ��Ӹ���֮��Ϊ2��l | ||
| C�� | ��Ӧ��ķ��Ӹ���֮��Ϊ2��3 | D�� | ��Ӧǰ��ԭ�ӡ����ӵĸ������� |
| �� �� | �� �� | �� �� �� �� | �� �� �� Ӧ |
| 101�桫102�� | 150�桫160�� ���� | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
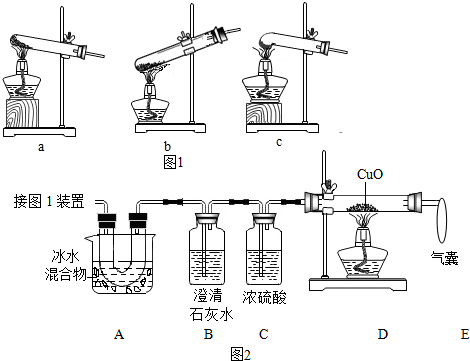
��2��ͼ2����֤�ȷֽ�����к� CO��CO2��װ�ã�
��װ��A����Ҫ�����dz�ȥ������������ֹ�Զ�����̼�ļ���������ţ�
�����ҵ��������ռ�δ��Ӧ��һ����̼����ֹ������Ⱦ��
��֤������CO2��������Bװ���ڵij���ʯ��ˮ����ǣ�B�з�Ӧ�Ļ�ѧ����ʽCO2+Ca��OH��2�TCaCO3��+H2O��
��֤������CO��������Dװ���к�ɫ�����죮
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��һ����̼û��ȫ��������ͭ��Ӧ����дһ�����ɣ�
��4����ȡ17.5g���ᾧ����Ʒ����50.00g��Һ����������ϡ���ᣬȻ��μ�KMnO4��Һ����KMnO47.9�ˣ�ǡ�÷�Ӧ��ȫ������֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
�������Ʒ�в��ᾧ�壨H2C2O4•2H2O ����������������д��������̣�
[�й����ʵ���Է���������Mr��H2C2O4��=90��Mr��H2C2O4•2H2O��=126��Mr��KMnO4��=158]��
| A�� | ������������Ԫ����� | |
| B�� | �������������6��̼ԭ�ӡ�13����ԭ�ӡ�1����ԭ�ӡ�2����ԭ�ӹ��� | |
| C�� | ��������̼Ԫ�ص������������ | |
| D�� | ������������� |
| A�� | ���顢������CH4 | B�� | �������ơ��ռNa0H | ||
| C�� | �����ơ���ʯ�ҡ�Ca��OH��2 | D�� | ̼�����ơ��մ�NaHCO3 |
| A�� | Mg | B�� | Cu | C�� | Fe | D�� | Na |
 ��ͼ��a��b���ֹ������ʵ��ܽ�����ߣ�
��ͼ��a��b���ֹ������ʵ��ܽ�����ߣ�