��Ŀ����
6�������ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�ⶨ�������е�̼��ƺ�����ijͬѧ��������ϴ������ָ��ﲢ���飬չ��������̽������1����20ml������������Ϊ35%��Ũ�������Ƴ�10%��ϡ���ᣬ��������Ϊ��
����1������20ml������������Ϊ35%��Ũ���������Ϊ24g��
��������������Ϊ35%��Ũ������ܶȦ�Ϊ1.2g/ml��
����2�������������ˮ�����Ϊ60ml��
����2�����ݼ�����������Ͳ�ֱ���ȡ�����ˮ��Ũ���
����3������ȡˮ�����ձ��У�Ȼ����ȡŨ���ᵹ��ˮ�в��ò���������õ�ϡ���ᣮ
��2����ü����ǡ����ձ���������16g�����������ձ��У������Ƶ�ϡ����ȫ�����루������������ò�������ֽ��裬ʹ���ַ�Ӧ��ֱ�����ٲ�������Ϊֹ�����輦�����е��������ʲ���ϡ���ᷴӦ����ʵ�����ݼ�¼ �����
| �����ǵ����� | ���ձ������� | ��ַ�Ӧ���ձ���������������� |
| 16g | 60g | 155.6g |
�ڼ���ü�������̼��Ƶ�����������
���� ��1����Һϡ���������ʵ��������ֲ��䣬��Һϡ����Ϊ���㡢���������裻
��2�����������غ㶨�ɿ�֪�������������ļ�������Ϊ�����˶�����̼�����Կ������������̼�����������ݶ�����̼�Ͷ�Ӧ�Ļ�ѧ����ʽ����̼��Ƶ����������������Ӧ������������
��� �⣺��1����20ml������������Ϊ35%��Ũ�������Ƴ�10%��ϡ���ᣬ��������Ϊ��
����1������20ml������������Ϊ35%��Ũ���������Ϊ20ml��1.2g/ml=24g��
����2�������������ˮ������Ϊx��
24g��35%=��24g+x����10%
x=60g�ۺ����Ϊ60mL��
����2�����ݼ��������� ��Ͳ�ֱ���ȡ�����ˮ��Ũ���
����3������ȡˮ�����ձ��У�Ȼ����ȡŨ���ᵹ��ˮ�в��ò���������õ�ϡ���ᣮ
��2�����������غ㶨�ɿ�֪���ɶ�����̼������Ϊ16g+60g+24g+60g-155.6g=4.4g��
�ڽ⣺��16g�������к���̼��Ƶ�����Ϊx��
CaCO3+2HCl�T�TCaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{44}$=$\frac{x}{4.4g}$
x=10g
�ü�������̼��Ƶ���������Ϊ$\frac{10g}{16g}$��100%=62.5%
�𣺸ü�������̼��Ƶ���������Ϊ62.5%��
����
��1��24�� 60�� ��Ͳ��
��2����ʵ�������ɶ�����̼������Ϊ 4.4g��
�ڸü�������̼��Ƶ���������62.5%��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������

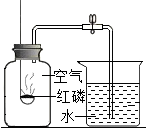 ��ͼװ�ó������ⶨ�����������ĺ��������жԸ�ʵ�����ʶ����ȷ���ǣ�������
��ͼװ�ó������ⶨ�����������ĺ��������жԸ�ʵ�����ʶ����ȷ���ǣ�������| A�� | ʹ�ú�����Խ�࣬���ս��뼯��ƿ��ˮҲԽ�� | |
| B�� | ȼ�ճ��еĺ����Ի���ϸ��˿ | |
| C�� | ��ʵ���˵��N2������ˮ | |
| D�� | ��ʵ������У�ȼ�ճ����뼯��ƿ���ٶ�̫������Ӱ��ⶨ�Ľ�� |
| A�� |  �μ�ҩƷ | B�� |  �������� | C�� |  Һ����� | D�� |  �����Լ�� |
| A�� | ���������ζ | B�� | �۲�������ɫ | C�� | �������ʯ��ˮ | D�� | ����ȼ��ľ�� |
 �����ʵ���Һ���������ʡ����Һ�ȣ����ܽ��������ͼ��ʾ������˵����ȷ���ǣ�������
�����ʵ���Һ���������ʡ����Һ�ȣ����ܽ��������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���ܽ�����¶ȵ����߶����� | |
| B�� | ��70��ı�����Һ�����¶ȣ��о������� | |
| C�� | 20��ʱ��100 g�ı�����Һ����������Ϊ28.6 g | |
| D�� | t��ʱ��������������Ϊ50%�ļ���Һ����tһ��С��70 |
