��Ŀ����
20��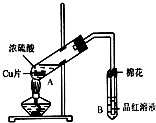 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о�����1��д���Թ�B�е�ʵ������Ʒ����Һ��ɫ��
��2��д��A�з�Ӧ�Ļ�ѧ����ʽCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O
��3��B�Թܿڵ���Ӧմ�е��Լ���NaOH��
��4����Ӧ����ͭʣ�࣬��������Ũ���ᣬҪʹͭƬ�ܽ⣬������A�Թ��м���H2O2������ͭƬ���ܽ⣬��Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H+=Cu2++2H2O�����߿��Լ�Fe2O3��NaNO3����д�������ڲ�ͬ������ʵĻ�ѧʽ����Ҳ��ʹͭƬ�ܽ⣮
��5��С���Ա��Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4•xH2O����С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ��
����������һ��ʵ������ݣ�
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
A������ͭ�����к��в��ӷ������� B��ʵ��ǰ���������ʪ��ˮ
C������ʱ�о���ɽ���ȥ D������ʧˮ��¶���ڿ�������ȴ��
���� ��1�������������Ư���ԣ���ʹƷ����Һ��ɫ��
��2�����������£�Cu��Ũ���ᷴӦ����CuSO4��SO2��H2O��
��3���������������������������ж�������ֱ���ſգ������ü�Һ���գ�
��4�����������£�˫��ˮ������Cu����ͭ���ӣ����Ρ����ᶼ������Cu��
��5���ᾧˮ����=��22.7-18.9��g=3.8g������ͭ����=��18.9g-11.7g��=7.2g��n��H2O����n��CuSO4��=$\frac{3.8g}{18g/mol}$��$\frac{7.2g}{160g/mol}$��4.7��5����ᾧˮ������ƫС��
A������ͭ�����к��в��ӷ������ʵ��½ᾧˮ����ƫС��
B��ʵ��ǰ���������ʪ��ˮ���½ᾧˮ����ƫ��
C������ʱ�о���ɽ���ȥ��Ӱ��ᾧˮ������
D������ʧˮ��¶���ڿ�������ȴ���½ᾧˮ����ƫС��
��� �⣺��1�������������Ư���ԣ���ʹƷ����Һ��ɫ�����Կ�����������Ʒ����Һ��ɫ���ʴ�Ϊ��Ʒ����Һ��ɫ��
��2�����������£�Cu��Ũ���ᷴӦ����CuSO4��SO2��H2O����Ӧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��3���������������������������ж�������ֱ���ſգ������ü�Һ���գ�������պȡ����ҺΪ��Һ����NaOH��Һ���ʴ�Ϊ��NaOH��Һ��
��4�����������£�˫��ˮ������Cu����ͭ���ӣ����ӷ���ʽΪCu+H2O2+2H+=Cu2++2H2O�����Ρ����ᶼ������Cu����Ӧ����Һ����ϡ����ʣ�࣬���Կ��Լ����������������ƣ��������ܽ����������������������������������������������Cu����ͭ�Σ�
�ʴ�Ϊ��Cu+H2O2+2H+=Cu2++2H2O��Fe2O3��NaNO3��
��5���ᾧˮ����=��22.7-18.9��g=3.8g������ͭ����=��18.9g-11.7g��=7.2g��n��H2O����n��CuSO4��=$\frac{3.8g}{18g/mol}$��$\frac{7.2g}{160g/mol}$��4.7��5����ᾧˮ������ƫС��
A������ͭ�����к��в��ӷ������ʵ��½ᾧˮ����ƫС������ȷ��
B��ʵ��ǰ���������ʪ��ˮ���½ᾧˮ����ƫ�ʴ���
C������ʱ�о���ɽ���ȥ����Ϊ��������ɷֺ;���ɷ���ͬ�����Բ�Ӱ��ᾧˮ�������ʴ���
D������ʧˮ��¶���ڿ�������ȴ����ˮ����ͭ����ˮ���½ᾧˮ����ƫС������ȷ��
�ʴ�Ϊ��ƫС��AD��
���� ������ͭ��Ũ���ᷴӦΪ���忼������ʵ�鷽����ƣ���ȷʵ��ԭ�������������ǽⱾ��ؼ����ѵ��ǣ�4����������Һ���ܽ�Cu�����ʵ�ѡȡ���״���������������Ŀ�Ѷ��еȣ�

| A�� | ͬ������ͬ�ܶȵ�C2H4��CO | B�� | ͬ�¶�ͬ�����C2H4��NO | ||
| C�� | ͬ�¶�ͬѹǿ��CO��N2 | D�� | ͬ���ͬѹǿ��N2��N2H4 |

| A�� | �缫�����ܽ� | B�� | �缫����������Ӧ | ||
| C�� | �������缫���A���缫�� | D�� | �缫��ĵ缫��Ӧ��Cu2++2e-=Cu |
| A�� | c��CH3COO -����c��CH3COOH�� | B�� | c��CH3COO -����c��Na+����c��H+����c��OH -�� | ||
| C�� | c��Na+��=c��CH3COO -��=0.01mol•L-1 | D�� | c��CH3COOH��+c��CH3COO -��=0.02mol•L-1 |
| A�� | 4��ʱ40mlˮ | B�� | 0.8mol���� | ||
| C�� | 9.03��1022�������� | D�� | 54g�� |
| A�� | 0.0025 mol•L-1 | B�� | 0.0001mol•L-1 | C�� | 0.001mol•L-1 | D�� | 0.005mol•L-1 |
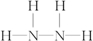 ��1mol����������ᷴӦ�����ӷ���ʽ��N2H4+2H+�TN2H62+ ����������ԭ��Ӧ����H2O2���ƣ��ȿ������������ֿ�����ԭ�������ݼ�̬�����������������ԭ����N2H4�е�Ԫ�صĻ��ϼ�Ϊ-2�ۣ��ȿ������ߣ��ֿ��Խ��ͣ�8gҺ̬����Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ��ų�375kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-1 500 kJ/mol��
��1mol����������ᷴӦ�����ӷ���ʽ��N2H4+2H+�TN2H62+ ����������ԭ��Ӧ����H2O2���ƣ��ȿ������������ֿ�����ԭ�������ݼ�̬�����������������ԭ����N2H4�е�Ԫ�صĻ��ϼ�Ϊ-2�ۣ��ȿ������ߣ��ֿ��Խ��ͣ�8gҺ̬����Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ��ų�375kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-1 500 kJ/mol��