��Ŀ����
4��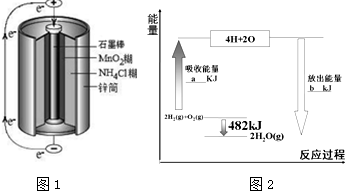 ��Ҫ��ش����и��⣺
��Ҫ��ش����и��⣺��1�������������ʣ��ٸɱ����ڽ��ʯ�������Ȼ�̼�����Ȼ��ƣ��ݶ������裻������þ�����ñ����д���пհף�
�����ۻ�ʱ��Ҫ�ƻ����ۼ��Ļ������Ǣ�
�������ڷ��Ӿ����ҷ��ӿռ乹��Ϊֱ���͵��Ǣ٣������ʽΪ

�����й��ۼ������ӻ������Ǣޣ�
��2���������н�������ǿ��Ԫ����Na����Ԫ�ط��ţ����������ڱ��е������ڵ�VA��Ԫ��ԭ�ӵĽṹʾ��ͼ
 ����������Ԫ���У��γɼ����Ӱ뾶��С����Al3+�������ӷ��ţ�
����������Ԫ���У��γɼ����Ӱ뾶��С����Al3+�������ӷ��ţ���3��п�̸ɵ��������ʹ�õĻ�ѧ��Դ�������������ͼ1��ʾ���õ�صĸ���������Zn������·��ͨ��0.4mole-��������������13g������ʱNH4+�������ŵ�����������壬����һ��������Ӻ�10e-�����������ĵ缫��ӦʽΪ2NH4++2e-�T2NH3��+H2����
��4����1mol��̬������ij�ֹ��ۼ���Ҫ���յ����������Ǹù��ۼ��ļ��ܣ��±���ijЩ���ۼ��ļ��ܣ�
| ���ۼ� | H-H | O=O | H-O |
| ����/kJ•mol-1 | 436 | 498 | X |
��ͼ�У�a=1370�� �ڱ����У�X=463��
���� ��1�������ۻ�ʱ��Ҫ�ƻ����ۼ��Ļ������Ƕ������裻
�������ڷ��Ӿ����ҷ��ӿռ乹��Ϊֱ���͵��Ƕ�����̼���Ӿ��壬������̼�ĵ���ʽΪ�� ��
��
�����Ȼ�����ֻ�����Ӽ��Ĺ��ۻ����������þ�Ǻ��й��ۼ������ӻ����
��2������Ԫ�������ɣ�ͬ����Ԫ�ش������ҽ������ڼ�����ͬ����������½�������ǿ�����ڱ��е������ڵ�VA��Ԫ������ԭ�ӽṹʾ��ͼΪ�� ��ͬ���������ӵİ뾶���������ӵİ뾶�����Ӳ���ͬ�������Ӻ˵����Խ��뾶ԽС��
��ͬ���������ӵİ뾶���������ӵİ뾶�����Ӳ���ͬ�������Ӻ˵����Խ��뾶ԽС��
��3��п�̸ɵ�صĸ�����Znʧȥ���ӣ���������1molʱת��2mol���ӣ�NH4+�����������ŵ����2�����壬����һ����������Ǻ�10e-����Ϊ��������һ��Ϊ������
��4���ٴ�ͼ����Կ�����a�ǶϿ�2mol H-H����1mol O=O��Ҫ���յ���������ϱ������ṩ�ļ��ܼ��ɼ������
�ڴӱ������Ͽ�2molH-H����1mol O=O�����ȣ�436��2+498��KJ�����H-O���ļ�����X�����γ�2molH2O�е�4molH-Oʱ��ų�4XKJ����������2molH2��g����1molO2��g������2molH2O��g��ʱ����482KJ����ʽ���ɽ��X��
��� �⣺��1�������ʯ�ۻ����ƻ����ۼ������ǵ��ʣ������������ۻ�ʱ��Ҫ�ƻ����ۼ��Ļ�����ʴ�Ϊ���ݣ�
��������̼���ڷ��Ӿ����ҷ��ӿռ乹��Ϊֱ���ͣ�������̼�ĵ���ʽΪ ���ʴ�Ϊ���٣�
���ʴ�Ϊ���٣� ��
��
�����Ȼ�����ֻ�����Ӽ��Ĺ��ۻ����������þ�Ǻ��й��ۼ������ӻ�����ʴ�Ϊ���ޣ�
��2������Ԫ�������ɣ�ͬ����Ԫ�ش������ҽ������ڼ�����ͬ����������½�������ǿ�����Զ������н�������ǿ��Ԫ����Ԫ�����ڱ������½ǵ�Na�����ڱ��е������ڵ�VA��Ԫ������ԭ�ӽṹʾ��ͼΪ�� ��ͬ���������ӵİ뾶���������ӵİ뾶�����Ӳ���ͬ�������Ӻ˵����Խ��뾶ԽС�����뾶��С�������������ӣ������ӷ���ΪAl3+���ʴ�Ϊ��Na��
��ͬ���������ӵİ뾶���������ӵİ뾶�����Ӳ���ͬ�������Ӻ˵����Խ��뾶ԽС�����뾶��С�������������ӣ������ӷ���ΪAl3+���ʴ�Ϊ��Na�� ��Al3+��
��Al3+��
��3��п�̸ɵ�صĸ�����Znʧȥ���ӣ�������ӦΪZn-2e-�TZn2+����������1molʱת��2mol���ӣ�ÿͨ��0.4mole-��������������0.2mol��65g/mol=13.0g����NH4+�����������ŵ����2�����壬����һ����������Ǻ�10e-����Ϊ��������һ��Ϊ������������ӦΪ2NH4++2e-�T2NH3��+H2����
�ʴ�Ϊ��Zn��13.0��2NH4++2e-�T2NH3��+H2����
��4���ٹ��ۼ��ļ��ܼ�Ϊ��1mol��̬������ij�ֹ��ۼ���Ҫ���յ���������ͼ����Կ�����a�ǶϿ�2mol H-H����1mol O=O��Ҫ���յ���������a=2mol��436kJ•mol-1+1mol��498kJ•mol-1=1370KJ���ʴ�Ϊ��1370��
�ڷ�Ӧ�Ȼ�ѧ����ʽ��2H2��g�� +O2��g�� =2H2O��g�� ��H=-482kJ•mol-1�����У�436��2+498��KJ-4XKJ=-482kJ�����X=463KJ���ʴ�Ϊ��463��
���� ���⿼�黯ѧ�����绯ѧ����ؼ��㣬�ڣ�4����Ӷϼ����ɼ��ĽǶȿ����˷�Ӧ�ȵ��йؼ��㣬���ڻ�������Ŀ���ϼ�

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�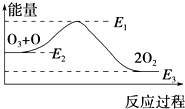 ��ԭ�Ӷ�O3�ķֽ��д����ã�O3+Cl�TClO+O2��H1��ClO+O�TCl+O2��H2��
��ԭ�Ӷ�O3�ķֽ��д����ã�O3+Cl�TClO+O2��H1��ClO+O�TCl+O2��H2������������ķֽⷴӦ�ǣ�O3+O�T2O2��H���÷�Ӧ�������仯��ͼ��ʾ��������������ȷ���ǣ�������
| A�� | ��ӦO3+O�T2O2�ġ�H=E1-E3 | B�� | ��ӦO3+O�T2O2�����ȷ�Ӧ | ||
| C�� | ��H=��H1+��H2 | D�� | ��ԭ��û�иı�O3�ֽⷴӦ������ |
| A�� | ��ȫ��Ӧ������O2ʣ�� | |
| B�� | ԭ���������C2H4��C2H2�������Ϊ1.9L | |
| C�� | ��ȫ��Ӧ������ˮ������Ϊ9 g | |
| D�� | ԭ���������CO��CH4�����֮��һ��Ϊ1��1 |
| A�� | ��Ba��OH��2��Һ�еμ�NaHSO4��Һ��ǡ��Ϊ���ԣ�Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| B�� | NH4HCO3��Һ�����KOHŨ��Һ���ȣ�NH4++OH- $\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| C�� | ϡ�����������м��Ӧ��3 Fe+8H++2 NO3-=3 Fe3++2 NO��+4 H2O | |
| D�� | KI��Һ��H2SO4�ữ��H2O2��Һ��ϣ�2 I-+H2O2+2H+=2H2O+I2 |
| A�� |  | B�� | N??N | C�� |  | D�� | 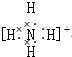 |
| A�� | HClO | B�� | HNO2 | C�� | H2SO4 | D�� | HIO4 |
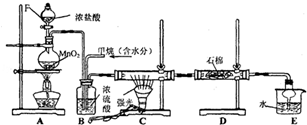
 ����ϳɹ������漰�ķ�Ӧ���ͷֱ��ǣ�������
����ϳɹ������漰�ķ�Ӧ���ͷֱ��ǣ�������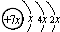 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺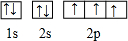
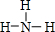 ��������ԭ��D���ӻ���ʽΪsp3��
��������ԭ��D���ӻ���ʽΪsp3��