��Ŀ����
2��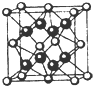 ��֪A��B��C��D��E��ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�B��C�γɵ�-1��������������Ϊ8���ӣ�D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ��ͼ����ش�
��֪A��B��C��D��E��ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�B��C�γɵ�-1��������������Ϊ8���ӣ�D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ��ͼ����ش���1��AԪ�ص��������⣻A��̼Ԫ���γ���Ļ������VSEPRģ��Ϊ�������壮
��2��B��A�γɵĻ������C��A�γɵĻ�����е�ߣ���ԭ���Ƿ�������Ӽ����������Ȼ�����Ӽ�û�������
��3��EԪ����Ԫ�����ڱ���λ��Ϊ�������ڵ�VIIB�壬����+2�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5��
��4����ͼ�п��Կ�����O������Ԫ��ΪCa����Ԫ�ط��ţ��������ӻ����ᄃ����ܶ�Ϊa g•cm-3�����������$\frac{78��4}{a{N}_{A}}$cm3��ֻҪ���г���ʽ��
���� A��B��C��D��E��ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ���A��HԪ�أ�
B��C�γɵ�-1��������������Ϊ8���ӣ���B��Cλ�ڵ�VIIA�壻
D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ���E��MnԪ�أ�D��ԭ��������EС5����D��CaԪ�أ�
B��Cԭ������С��D������B��FԪ�ء�C��ClԪ�أ��ٽ����Ŀ�������
��� �⣺A��B��C��D��E��ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ���A��HԪ�أ�
B��C�γɵ�-1��������������Ϊ8���ӣ���B��Cλ�ڵ�VIIA�壻
D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ���E��MnԪ�أ�D��ԭ��������EС5����D��CaԪ�أ�
B��Cԭ������С��D������B��FԪ�ء�C��ClԪ�أ�
��1��A��HԪ�أ�A��̼Ԫ���γ���Ļ������Ǽ��飬���������Cԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ����Լ����VSEPRģ��Ϊ��������ṹ��
�ʴ�Ϊ���⣻�������壻
��2��A��B��CԪ�طֱ���H��F��ClԪ�أ�HF�к��������HCl�в������������Ĵ��ڵ����⻯����۷е����ߣ�����HF���۷е����HCl���ʴ�Ϊ����������Ӽ����������Ȼ�����Ӽ�û�������
��3��E��MnԪ�أ������ڱ���λ�ڵ������ڵ�VIIB�壬ʧȥ�����2�������γɶ��������ӣ�
����+2�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5��
�ʴ�Ϊ���������ڵڢ�B�壻1s22s22p63s23p63d5��
��4���þ����к�ɫ�������8����ɫ�����=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����Ժ�ɫ��Ͱ�ɫ�����֮��=8��4=2��1����Ϊ������Ϊ-1�ۡ�������Ϊ+2�ۣ��������з�����������Ӹ���֮��Ϊ2��1�����ԡ�O������Ԫ��ΪCa���þ������=$\frac{\frac{M}{{N}_{A}}��4}{��}$=$\frac{78��4}{a{N}_{A}}$cm3���ʴ�Ϊ��Ca��$\frac{78��4}{a{N}_{A}}$��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ�Ӻ�������Ų������ӿռ乹���жϵ�֪ʶ�㣬��ȷ��̯��������ԭ�����۲���ӶԻ������ۼ��ɽ���ѵ��Ǿ������㣮

 ��У����ϵ�д�
��У����ϵ�д��� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |

��2���ؿ��к�������Ԫ����O��
��3������ܵĵ����ڼ��������·�Ӧ����һ�ֵ���ɫ���壬�ù���ĵ���ʽΪ
 ��
����4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����NaOH�������Ե�����������Al��OH��3������������ʻ�ѧʽ��
��5��д���ݵĵ���������������Һ��Ӧ�����ӷ���ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
| A�� | �����˻�ѧ��Ӧ���ʱ� | |
| B�� | ������ͬ�ȳ̶ȵؼӿ����淴Ӧ���� | |
| C�� | �����˷�Ӧ����Ļ�� | |
| D�� | ø���и߶ȵĴ����Ժ�רһ�� |
| A�� | ���ӻ������п��ܺ��й��ۼ� | |
| B�� | �ڹ��ۻ�������һ�����й��ۼ� | |
| C�� | �����Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� |
| A�� | ��������NaOH���壬ƽ�������ƶ�����Һ��c��H+������ | |
| B�� | ��ˮ��ƽ�������ƶ���$\frac{c��C{H}_{3}COOH��}{c��C{H}_{3}CO{O}^{-}��}$��С | |
| C�� | ͨ������HCl��ƽ�������ƶ�����Һ��c��H+������ | |
| D�� | ��������CH3COONa���壬ƽ�������ƶ�����Һ������������ |
| A�� | �κ�һ��������ԭ��Ӧ���������Ϊԭ��أ�������� | |
| B�� | �����£���pH=2�������pH=12�İ�ˮ�������Ϻ���Һ�ʼ��ԣ�c��NH4+����c��Cl-����c��OH-����c��H+�� | |
| C�� | �κο��淴Ӧ����ƽ�ⳣ��Խ��Ӧ���ʡ���Ӧ���ת����Խ�� | |
| D�� | pH=9��CH3COONa��Һ��pH=9��NH3•H2O��Һ������Һ��ˮ�ĵ���̶���ͬ |
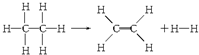
���ڷ�Ӧ��������1mol���飨��Ӧ������йظ÷�Ӧ��˵����ȷ���ǣ�������
| A�� | �÷�Ӧ�ų�251.2 kJ������ | B�� | �÷�Ӧ����251.2 kJ������ | ||
| C�� | �÷�Ӧ�ų�125.6 kJ������ | D�� | �÷�Ӧ����125.6 kJ������ |
| A�� | X��Y��Z | B�� | Y��X��Z | C�� | X��Z��Y | D�� | Y��Z��X |
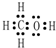 ��Y������ԭ���ӻ���ʽΪSP2��
��Y������ԭ���ӻ���ʽΪSP2��