��Ŀ����
11����ͼ����CO2�����ԭΪ̼�⻯����Ĺ���ԭ��ʾ��ͼ����һ�ּ���ˮ��Һ�����Һ��ͼ������H2��ԭCO2�Ʊ��״��Ĺ���ԭ��ʾ��ͼ������Ϊ�������Һ������˵������ȷ���ǣ�������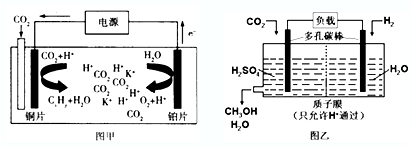
| A�� | ����ͭƬ��������K+��ͭƬ�缫�ƶ� | |
| B�� | ������CxHyΪC2H4��������1 mol C2H4��ͬʱ����2 molO2 | |
| C�� | ����H2SO4����������ǿ��Һ�ĵ����� | |
| D�� | �������������ĵ缫��ӦΪCO2+6e-+6H+�TCH3OH+H2O |
���� ��ͼ�У�CO2�����ԭΪ̼�⻯����ɵ��������֪����ͨ��CO2��һ��Ϊ���ص���������һ��Ϊ����������������������ʧ����������������ͼ�У�ȼ�ϵ�ص����������������õ��ӵĻ�ԭ��Ӧ��
��� �⣺A�����ʱ�����������ǣ�������������������������ͭΪ����������������K+��ͭƬ�缫�ƶ�����A��ȷ��
B����CxHyΪC2H4��������ܷ�ӦΪ��2CO2+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$3O2+C2H4����������1 mol C2H4��ͬʱ����3 molO2����B����
C�����е������ҺH2SO4����������ǿ�����ԣ���C��ȷ��
D����ͼ��֪�����������Ƕ�����̼�����õ��ӵĻ�ԭ��Ӧ�Ĺ��̣������ĵ缫��ӦΪCO2+6e-+6H+�TCH3OH+H2O����D��ȷ��
��ѡB��
���� ���⿼��ѧ�����صĹ���ԭ��֪ʶ��������ѧ���ķ��������Ŀ��飬ע��������Ӧʽ����д�Լ�������ԭ��Ӧ֮��Ĺ�ϵ��ע����ɵ��ܽ��ǹؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
1�������й�ԭ�ӽṹ��Ԫ�������ɱ�����ȷ���ǣ�������
| A�� | ��A��Ԫ����ͬ�����зǽ�������ǿ��Ԫ�� | |
| B�� | ԭ������Ϊ15��Ԫ�ص�����ϼ�Ϊ+3 | |
| C�� | ������������2��Ԫ��һ��λ��Ԫ�����ڱ��ĵڢ�A�� | |
| D�� | �ڶ����ڢ�A��Ԫ�ص�ԭ�Ӻ˵������������һ��Ϊ6 |
2��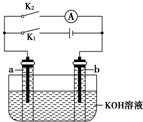 ��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ���ǣ�������
��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ���ǣ�������
 ��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ���ǣ�������
��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ���ǣ�������| A�� | �Ͽ�K2���պ�K1һ��ʱ�䣬��Һ��pH��� | |
| B�� | �Ͽ�K1���պ�K2ʱ��b���ϵĵ缫��ӦʽΪ��2H++2e-�TH2�� | |
| C�� | �Ͽ�K2���պ�K1ʱ��a���ϵĵ缫��ӦʽΪ��4OH--4e-�TO2��+2H2O | |
| D�� | �Ͽ�K1���պ�K2ʱ��OH-��b���ƶ� |
6�����и�ʵ������У����ܹ۲첻����Һ��������������ǣ�������
| A�� | ����ͨ�������� | B�� | H2S����ͨ�뱥����ˮ | ||
| C�� | H2S����ͨ�������� | D�� | SO2����ͨ�������� |
16������������͢����ȷ���������ϵ���ǣ�������
| ѡ�� | ����I | ����II |
| A | ��������ܽ�����¶����߶����� | ������Ũ������ȴ�ᾧ�����ˣ���ȥ�������л��е���ɳ |
| B | NH4Cl�������ֽ� | ����NH4Cl�Ʊ�NH3 |
| C | SO2����Ư���� | SO2��ʹKMnO4��Һ��ɫ |
| D | ij�¶��£�̼���K1=4.4��10-7���������K=2.98��10-8 | ��CO2��NaClO�Ʊ�HClO |
| A�� | A | B�� | B | C�� | C | D�� | D |
3���ִ�ұ�������̵�һ�ֹ�����������ͼ��ʾ��
�±�Ϊt��ʱ���й����ʵ�pKp��ע��pKp=-1gKp��
�����̿�ԭ�����ķ�ӦΪ��12MnO2+C6H12O6+12H2SO4=12MnSO4+6CO2��+18H2O�÷�Ӧ�У���ԭ��ΪC6H12O6��д��һ�������ԭ�������ʵĴ�ʩ�������¶Ȼ����̿���ϸ��
����Һ1��pH����������������T����MnSO4����Һ��pH��
�ۼ���MnF2��Ŀ�ij�ȥCa2+���Ca2+������Fe3+������Cu2+����

�±�Ϊt��ʱ���й����ʵ�pKp��ע��pKp=-1gKp��
| ���� | Fe��OH��3 | Cu��OH��2 | Ca��OH��2 | Mn��OH��2 | CuS | CaS | MnS | MnCO3 |
| pKp | 37.4 | 19.3 | 5.26 | 12.7 | 35.2 | 5.86 | 12.6 | 10.7 |
����Һ1��pH����������������T����MnSO4����Һ��pH��
�ۼ���MnF2��Ŀ�ij�ȥCa2+���Ca2+������Fe3+������Cu2+����
 �ܱ�������mA��g��+nB��g��?pC��g������Ӧ�ﵽƽ��״̬�����ⶨ����ѹǿpʱ��A��ת������p���仯��������ͼ��ʾ��
�ܱ�������mA��g��+nB��g��?pC��g������Ӧ�ﵽƽ��״̬�����ⶨ����ѹǿpʱ��A��ת������p���仯��������ͼ��ʾ��