��Ŀ����
3���ִ�ұ�������̵�һ�ֹ�����������ͼ��ʾ��
�±�Ϊt��ʱ���й����ʵ�pKp��ע��pKp=-1gKp��
| ���� | Fe��OH��3 | Cu��OH��2 | Ca��OH��2 | Mn��OH��2 | CuS | CaS | MnS | MnCO3 |
| pKp | 37.4 | 19.3 | 5.26 | 12.7 | 35.2 | 5.86 | 12.6 | 10.7 |
����Һ1��pH����������������T����MnSO4����Һ��pH��
�ۼ���MnF2��Ŀ�ij�ȥCa2+���Ca2+������Fe3+������Cu2+����
���� �����̿���Ҫ�ɷ�ΪMnCO3�������������ơ�ͭ�������ʣ��м�������ͻ�ԭ�������̿�ԭ�����ķ�ӦΪ��12MnO2+C6H12O6+12H2SO4=12MnSO4+6CO2��+18H2O����ԭ����Һ�к���Mn2+��Ca2+��Fe3+��Cu2+��Fe��OH��3pKpΪ37.4������������������pH���Խ�����������������������Һ�м�����泥�CuSpKpΪ35.2�����Խ�ͭ�����γ���������������ټ���MnF2���γ�CaF2��������ȥCa2+�����Եõ��ĺ��������ӵ��ε�⣬���Եõ������̣�
���ڻ�ѧ��Ӧ��Ԫ�ػ��ϼ����ߵ��ǻ�ԭ�������ϼ۽��͵�Ϊ��������Ӱ������������ʵ��������¶ȡ�Ũ�ȡ���������ܼ������أ�
��KpΪ����ƽ�ⳣ����pKp=-1gKp��pKpԽ�����ܽ�ƽ�ⳣ��ԽС����Һ1Ϊ�γ�Fe��OH��3���������Һ���ɷ�ΪCa2+��Cu2+��Mn2+������pKp�жϳ���Һ1PH����MnSO4����Һ��pH��
��CaF2������ˮ������MnF2��Ŀ�ij�ȥ�γ�CaF2��������ȥCa2+��
��� �⣺�����̿���Ҫ�ɷ�ΪMnCO3�������������ơ�ͭ�������ʣ��м�������ͻ�ԭ�������̿�ԭ�����ķ�ӦΪ��12MnO2+C6H12O6+12H2SO4=12MnSO4+6CO2��+18H2O����ԭ����Һ�к���MnSO4��Ca2+��Fe3+��Cu2+��Fe��OH��3pKpΪ37.4������������������pH���Խ�����������������������Һ�м�����泥�CuSpKpΪ35.2�����Խ�ͭ�����γ���������������ټ���MnF2���γ�CaF2��������ȥCa2+�����Եõ��ĺ��������ӵ��ε�⣬���Եõ������̣�
��C6H12O6+12 MnO2+12H2SO4=12 MnSO4ʮ6CO2ʮ18H2O��Ӧ��C6H12O6�е�C��0�����ߵ�CO2�е�+4�ۣ�MnO2�е�Mn��+4�۽��͵�MnSO4��+2�ۣ�1molC6H12O6��Ӧ̼ԭ�ӹ�ʧȥ24�����ӣ�Ԫ�ػ��ϼ����ߵ��ǻ�ԭ����C6H12O6�е�C��0�����ߵ�CO2�е�+4�ۣ����Ի�ԭ��ΪC6H12O6��Ӱ������������ʵ��������¶ȡ�Ũ�ȡ���������ܼ������أ������¶Ȼ����̿���ϸ�ȴ�ʩ�����ԭ�������ʣ�
�ʴ�Ϊ��C6H12O6�������¶Ȼ����̿���ϸ�ȣ�
�����̿�ԭ�����ķ�ӦΪ��12MnO2+C6H12O6+12H2SO4=12MnSO4+6CO2��+18H2O����Һ��ǿ���ԣ���ԭ����Һ�к���Mn2+��Ca2+��Fe3+��Cu2+����ʱδ�γɳ�����KpΪ����ƽ�ⳣ����pKp=-1gKp��pKpԽ�����ܽ�ƽ�ⳣ��ԽС����Һ1Ϊ�γ�Fe��OH��3������pKp=-1gKp=37.4��Kp=10-37.4��Kp=c��Fe3+����c3��OH-��=10-37.4��c��OH-����10-10��c��H+��=1��10-4mol/L��pH=4��������Һ1��pH����MnSO4����Һ��pH=4�����γ���������������
�ʴ�Ϊ������
��CaF2������ˮ����Һ2ΪMn2+��Ca2+��NH4+��SO42-������MnF2��Ŀ�ij�ȥ�γ�CaF2��������ȥCa2+��
�ʴ�Ϊ��Ca2+��
���� ���⿼�����ִ�ұ�������̵�һ�ֹ������̷�����ע������ܽ�ƽ��Ӧ���Լ������з����ᴿ���ʵ�ԭ������Ŀ�Ѷ��еȣ�

| A�� | H+��Cu2+��Fe3+��SO4 2- | B�� | Ba2+��Na+��Al3+��Cl- | ||
| C�� | K+��Ag+ NH4+��NO3- | D�� | Na+��K+��Br-��OH- |
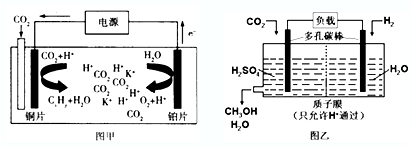
| A�� | ����ͭƬ��������K+��ͭƬ�缫�ƶ� | |
| B�� | ������CxHyΪC2H4��������1 mol C2H4��ͬʱ����2 molO2 | |
| C�� | ����H2SO4����������ǿ��Һ�ĵ����� | |
| D�� | �������������ĵ缫��ӦΪCO2+6e-+6H+�TCH3OH+H2O |
�������¶� �ڼ��������� ������Ӧ��Ũ�� �ܽ���״���巴Ӧ��ĥ�ɷ�ĩ��
| A�� | ֻ�Т٢ڢ� | B�� | ֻ�Т٢ڢ� | C�� | ֻ�Тۢ� | D�� | �٢ڢۢ� |
| A�� | pH=1����Һ��Na+��NH4+��S2-��NO3- | |
| B�� | c��HSO4-��=0.1 mol/L����Һ��K+��Ba2+��HCO3-��Cl- | |
| C�� | ����������������ɫ����Һ��Na+��K+��I-��SO42- | |
| D�� | ���ܽ�̼��Ƶ���Һ��Na+��NH4+��Cl-��Br- |
| �������� | �� �� | |
| A | KI������Һ�е�����ˮ��������ͨ��SO2����ɫ��ȥ | SO2����Ư���� |
| B | ����SnCl2��Һʱ���Ƚ�SnCl2��������ϡ���ᣬ��������ˮϡ�ͣ�������Լ�ƿ�м������������� | ����Sn2+ˮ�⣬����ֹSn2+������ΪSn4+ |
| C | ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����˵������Һ��һ������SO42- |
| D | ��Ũ�Ⱦ�Ϊ0.1mol•L-1��MgCl2��CuCl2�����Һ����μ��백ˮ������������ɫ���� | Ksp[Cu��OH��2]��Ksp[Mg��OH��2] |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Ũ���� | B�� | ϡ���� | C�� | Ũ���� | D�� | Ũ���� |
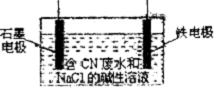 ������������ͭ���仯���������������й�ϵ���У�
������������ͭ���仯���������������й�ϵ���У�