��Ŀ����
8����1��25��ʱ����pH=5��������Һϡ��500����ϡ�ͺ���Һ�е�[SO42-]��[H+]ԼΪ1��10��2��25��ʱ��ijǿ����ҺpH=a��ijǿ����ҺpH=b����֪a+b=12�������Һ��Ϻ�pH=7��������Һ���V���ᣩ�ͼ���Һ���V�������ȷ��ϵΪV���=100V���ᣩ
��3��25��ʱ��pH=3��������ˮ�����H+��Ũ��Ϊ10-11mol•L-1
��4��ij�¶ȣ�t�棩ʱ�����0.01mol•L-1 ��NaOH��Һ��pH=11������¶���ˮ��Kw=10-13��mol/L��2�ڴ��¶���ij��Һ��pH=3���ڸ���Һ��[H+]��[OH-]=107��
��5����ˮ��[H+]=5.0��10-7 mol/L�����ʱ��ˮ�е�[OH-]=5.0��10-7mol/L��
���� ��1��ϡ��������������ʵ������䣬����Һ�ӽ�����ʱҪ����ˮ�ĵ��룬���Խ�pH=5��������Һϡ��500������Һ�е�SO42-��H+Ũ�ȵı�ֵԼΪ1��10��
��2��25��ʱ��Kw=10-14��ijǿ����ҺpH=a���������Һ��������Ũ��Ϊ10-a mol/L��ǿ����ҺpH=b�������Һ������������Ũ��=10 b-14 mol/L����Һ������˵�������Ӻ����������ӵ����ʵ�����ȣ�
��3��pH=3��������ˮ�����H+��Ũ�Ⱥ�ˮ�������������Ũ����ȣ�
��4��������Һ��pH������Һ��������Ũ�ȣ������������Ƶ�Ũ�ȼ�������������Ũ�ȣ��ٽ��Kw=c��H+����c��OH-�����㣻����c��OH-��=$\frac{Kw}{C��{H}^{+}��}$����$\frac{C��{H}^{+}��}{C��O{H}^{-}��}$ֵ��
��5�����ݴ�ˮ��c��OH-��=c��H+������Һ��ʾ���Խ��з�����
��� �⣺��1��pHΪ5����Һ��������Ũ��Ϊ��c��H+��=1��10-5mol/L����������ӵ�Ũ��Ϊ��c��SO42-��=5��10-6mol/L��
��Һϡ��500����������Ũ�Ȳ�����С��1��10-7mol/L��ֻ�����ӽ�1��10-7mol/L�������������Ũ��Ϊ��c��SO42-��=1��10-8mol/L��
����ϡ�ͺ���Һ�������������������Ũ��֮��Ϊ��1��10-8mol/L��1��10-7mol/L=1��10��
�ʴ�Ϊ��1��10��
��2��25��ʱ��Kw=10-14��ijǿ����ҺpH=a���������Һ��������Ũ��Ϊ10-a mol/L��ǿ����ҺpH=b�������Һ������������Ũ��=10 b-14 mol/L����Һ������˵�������Ӻ����������ӵ����ʵ�����ȣ���������ΪV���ᣩ��������ΪV�����10-a mol/L��V���ᣩL=10 b-14 mol/L��V���L����V1=10 a+b-14 V�������a+b=12����V���=100V���ᣩ��
�ʴ�Ϊ��V���=100V���ᣩ��
��3��pH=3������������������������ˮ�ĵ��룬c��OH-��=$\frac{Kw}{C��{H}^{+}��}$=$\frac{1{0}^{-14}}{1{0}^{-3}}$=10-11mol/L������Һ��ˮ�����H+��Ũ�Ⱥ�ˮ�������������Ũ����ȣ�
�ʴ�Ϊ��10-11��
��4��0.01mol•L-1��NaOH��Һ��pHΪ11����������Ũ��=10-11 mol/L������������ǿ�������ȫ���룬������Һ������������Ũ����0.01mol/L����Kw=c��H+����c��OH-��=0.01��10-11=10-13��
���¶���Kw=10-13��pH=3��Һ��C��H+��=10-3mol•L-1��c��OH-��=$\frac{Kw}{C��{H}^{+}��}$=$\frac{1{0}^{-13}}{1{0}^{-3}}$=10-10mol/L������$\frac{C��{H}^{+}��}{C��O{H}^{-}��}$=107��
�ʴ�Ϊ��10-13��mol/L��2��107��
��5����ˮ��ʾ���ԣ�c��OH-��=c��H+��=5.0��10-7mol/L���ʴ�Ϊ��5.0��10-7mol/L��
���� ���⿼�������ӻ��������йؼ��㣬������Һ������Լ�ˮ�����ӻ��������������ע��������Һ�������ӡ�����������Ũ������ҺpH�Ĺ�ϵ��ע��ˮ�����ӻ��������ȶ��Ĺ�ϵΪ�״��㣬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�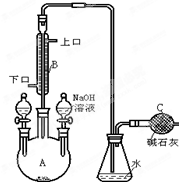 �Ʊ�һ���������ʵ��װ����ͼ��ʾ�����в�����ȷ���ǣ�������
�Ʊ�һ���������ʵ��װ����ͼ��ʾ�����в�����ȷ���ǣ�������| A�� | װ������Һ©���е�ҩƷ�DZ���Һ�� | |
| B�� | �Ҳ��Һ©���е����ʵ������Ǵ��� | |
| C�� | ������ƿ�м��������ữ��AgNO3��Һ���е���ɫ����������˵���˷�ӦΪȡ����Ӧ | |
| D�� | ����B����ƿ֮������һ�� װ�ò�����CCl4Һ������ȥHBr�������� װ�ò�����CCl4Һ������ȥHBr�������� |
| A�� | ͨ��ˮ�� | B�� | ͨ�����ʯ��ˮ�� | ||
| C�� | ͨ����ˮ | D�� | �����������й��� |
| A�� | ����NA����ԭ�ӵĵ����ڱ�״���µ����ԼΪ11.2L | |
| B�� | 25�棬1.01��105Pa��64gSO2�к��е�ԭ����С��3NA | |
| C�� | �ڳ��³�ѹ�£�11.2L Cl2���еķ�����Ϊ0.5NA | |
| D�� | 1 mo1 Na2O2������H2O��Ӧ��ת����2 NA ������ |
| A�� | ƽ��������Ӧ�����ƶ� | B�� | x+y��z | ||
| C�� | B��ת������� | D�� | C������������� |
| A�� | 0.1mol/LH2SO4��0.2mol/L ��ˮ��50mL | |
| B�� | 0.1mol/LCH3COOH��0.1mol/LNaOH ��Һ��50mL | |
| C�� | pH=1��CH3COOH��pH=13�� NaOH ��Һ��50mL | |
| D�� | pH=1�������� pH=13�� NaOH ��Һ��50mL |
| A�� | ľ����ά��������������ˮ������ɫ | |
| B�� | ʳ�û����ͺͼ����嶼�ܷ���ˮ�ⷴӦ | |
| C�� | ��װ�ò��Ͼ���ϩ�;�����ϩ�������� | |
| D�� | PX��Ŀ����Ҫ��Ʒ�Զ��ױ����ڱ����� |
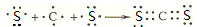 ��
��