��Ŀ����
17������������ͭ��Ӧ�Ļ�ѧ����ʽΪ��3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O��������м��㣺��1��0.45mol CuO��ȫ��Ӧ���ɵ����ڱ�״���µ����Ϊ3.36L
��2��3mol NH3��3mol CuO��Ӧ����1mol N2���������İ��������ɵ�ˮ��Ϊ��ˮ������ð�ˮ�а�����������������һλС������д����Ҫ�ļ�����̣�
���� ��1�����ݷ���ʽ�������ɵĵ��������ʵ������ٸ���V=nVm���㣻
��2�����ݷ���ʽ���㷴Ӧ���ĵİ��������ɵ�ˮ��Ȼ�����ʣ��İ���������������$\frac{m��N{H}_{3}��}{m��N{H}_{3}��+m��{H}_{2}O��}$���㣮
��� �⣺��1����֪3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O��0.45mol CuO��ȫ��Ӧ���ɵ���Ϊ0.45mol��$\frac{1}{3}$=0.15mol�����������V=nVm=0.15mol��22.4L/mol=3.36L��
�ʴ�Ϊ��3.36��
��2��3mol NH3��3mol CuO��Ӧ����1mol N2���ɷ���ʽ��֪��Ӧ���ĵİ���Ϊ2mol����ʣ��İ���Ϊ1mol����Ӧ���ɵ�ˮ�����ʵ���Ϊn��H2O��=3n��N2��=3mol��
��ʣ�ఱ��������Ϊm=nM=1mol��17g/mol=17g��m��H2O��=nM=3mol��18g/mol=54g��
�ð�ˮ�а�����������Ϊ$\frac{m��N{H}_{3}��}{m��N{H}_{3}��+m��{H}_{2}O��}$��100%=$\frac{17}{17+54}$��100%=23.9%��
�𣺸ð����а�����������Ϊ23.9%��
���� ���⿼���йط���ʽ�ļ��㣬�ѶȲ���ע����ݷ�Ӧ����ʽ�����ʵļ�����ϵ���н��

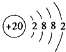
| ��Һ�����ʵ���Ũ��/mol•L-1 | �����/% | H+�����ʵ���Ũ��/mol•L-1 | pH |
| 0.1 | 2�� | c1 | x |
| c | �� | 2c1 | 3 |
| A�� | 14g | B�� | 47g | C�� | 54g | D�� | 94g |
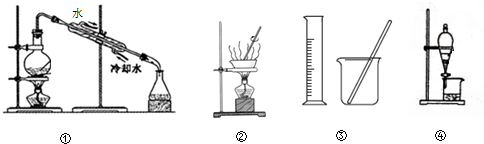
| A�� | �âٷ���NaCl��Һ��NaOH��Һ | |
| B�� | �â�����NH4Cl�ͱ�����Һ��ȡNH4Cl���� | |
| C�� | �â�����100mL 0.1000mol•L-1 K2Cr2O2��Һ | |
| D�� | �âܷ���Na2CO3��Һ��CH3COOC2H5 |
 ��
�� ��
��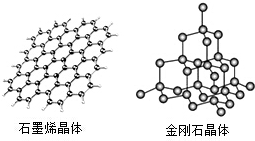 ̼���仯����㷺��������Ȼ���У��ش��������⣺
̼���仯����㷺��������Ȼ���У��ش��������⣺