��Ŀ����
6�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO��SiO2�����ʣ���ҵ�������̿���ȡMnSO4•H2O���������£�
��֪���ٲ��ֽ�����������ȫ����ʱ��pH���±�
| ���������� | Fe3+ | Al3+ | Mn2+ | Mg2+ |
| ��ȫ����ʱ��PHֵ | 3.2 | 5.2 | 10.4 | 12.4 |
��1����������������MnO2ת��ΪMn2+�����ӷ���ʽΪSO2+MnO2�TMn2++SO42-��
��2����1�������м���H2O2��Ŀ���ǽ�Fe2+����ΪFe3+��
��3����1���������γ�����l����Ҫ�ɷ�ΪAl��OH��3��Fe��OH��3���ѧʽ������pH��5-6���ӵ��Լ�����ѡ��a��b���������Լ��������ĸ���� a��Cao b��MgO c��Al2O3 d����ˮ
��4����2�����ӣ���Ҫ�ǽ�Ca2+��Mg2+ת��Ϊ��Ӧ�����������ȥ��д��MnF2��ȥMg2+�����ӷ�Ӧ����ʽMnF2+Mg2+�TMn2++MgF2���÷�Ӧ��ƽ�ⳣ����ֵΪ7.2��107��
����֪��MnF2��Ksp=5.3��10-3��CaF2��Ksp=1.5��10-10��MgF2��Ksp=7.4��10-11����
��5�����á����ȹ��ˡ�������ԭ������Ϊ����MnSO4•H2O��ˮ�е��ܽ⣬�õ������ò�Ʒ��
��6��ȡ����MnSO4•H2O����ˮ�������Һ������pH���ָ���Һ�����ԣ�ԭ����Mn2++2H2O?Mn��OH��2+2H+�������ӷ���ʽ��ʾ��������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊc��SO42-����c��Mn2+����c��H+����c��OH-����
���� �����̿�֪�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO�����ʣ��������ܽ����SO2+MnO2�TMnSO4������pH������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3��Ȼ���ȥ�����ӣ���ϱ������ݿ�֪CaF2���ܶȻ���С���Ҳ����������ʼ�MnF2���������Ũ�������ȹ��ˣ���ֹ����MnSO4•H2O�ܽ�����٣����Դ������
��� �⣺�����̿�֪�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO�����ʣ��������ܽ����SO2+MnO2�TMnSO4������pH������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3��Ȼ���ȥ�����ӣ���ϱ������ݿ�֪CaF2���ܶȻ���С���Ҳ����������ʼ�MnF2���������Ũ�������ȹ��ˣ���ֹ����MnSO4•H2O�ܽ�����٣���
��1������FeSO4�ڷ�Ӧ�����½�MnO2��ԭΪMnSO4��Fe2+������ΪFe3+���ʡ�������������MnO2ת��ΪMn2+�����ӷ���ʽΪSO2+MnO2�TMn2++SO42-���ʴ�Ϊ��SO2+MnO2�TMn2++SO42-��
��2����������������������������������ӣ��ʴ�Ϊ����Fe2+����ΪFe3+��
��3������pH��5��6������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3�����ӹ����в������������ʣ����Կɼ������ƺ�����þ������Һ��PH���ʴ�Ϊ��Al��OH��3��Fe��OH��3��a��b��
��4�����������������д���ӷ���ʽ�û�ѧʽ����Ӧ����ʽΪ��MnF2+Mg2+�TMn2++MgF2��K=$\frac{c��M{n}^{2+}��}{c��M{g}^{2+}��}$=$\frac{c��M{n}^{2+}����{c}^{2}��{F}^{-}��}{c��M{g}^{2+}����{c}^{2}��{F}^{-}��}$=$\frac{5.3��1{0}^{-3}}{7.4��1{0}^{-11}}$=7.2��107���ʴ�Ϊ��MnF2+Mg2+�TMn2++MgF2��7.2��107��
��5���¶ȸ���27��ʱ��MnSO4������ܽ�����¶ȵ����߶����ͣ����Բ��ó��ȹ��ˣ�����MnSO4•H2O��ˮ�е��ܽ⣬�ʴ�Ϊ����Ϊ����MnSO4•H2O��ˮ�е��ܽ⣬�õ������ò�Ʒ��
��6��MnSO4��ǿ�������Σ�ˮ������ԣ�����ʽΪ��Mn2++2H2O?Mn��OH��2+2H+������Ũ�ȴ�СΪ����ˮ�����ӣ�ˮ������ӣ����Ե����ӣ����Ե����ӣ���������Ũ�ȴ�СΪ��c��SO42-����c��Mn2+����c��H+����c��OH-�����ʴ�Ϊ��Mn2++2H2O?Mn��OH��2+2H+��c��SO42-����c��Mn2+����c��H+����c��OH-����
���� ���⿼����������ᴿ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�������̷������������뷽���������ķ�ӦΪ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | M=N | B�� | M��N | C�� | M��N | D�� | ���Ƚ� |
| A�� | NO2 | B�� | Fe��OH��3 | C�� | FeCl2 | D�� | H2SiO3 |
���۵�1070�棬������ˮ��ˮ��Һ����
���۵�10.34�棬Һ̬�����磬ˮ��Һ����
��������CS2���۵�112.8�棬�е�444.6��
���۵�97.81�棬�������磬�ܶ�Ϊ0.97g•cm-3��
| A�� | �����Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| B�� | ��ԭ�Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| C�� | �����Ӿ���ڷ��Ӿ���۽�������ܽ������� | |
| D�� | ��ԭ�Ӿ�������Ӿ���۷��Ӿ���ܷ��Ӿ��� |

����������������������ʽ����ʱ��pH���£�
| ������ | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| Fe��OH��2 | 7.6 | 9.7 |
| Ai��OH��2 | 7.1 | 9.2 |
��2���������ʱ�����������H2SO4 ���ѧʽ�������������a���������ٺ���Һ�п��ܺ��еĽ���������Ni2+��Fe2+��Fe3+��
��3������H2O2ʱ������Ӧ�����ӷ���ʽΪH2O2+2Fe2++2H+=2Fe3++2H2O��
��4������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��3.2-7.1��
��5����Ʒ��������ʱ����������̷���FeSO4•7H2O������ԭ�������H2O2���������㣨��H2O2ʧЧ��������ʱ�䲻�㵼��Fe2+δ����ȫ������ɵģ�д��һ�㼴�ɣ���
��6��NiS04•7H20�������Ʊ������أ�NiMH����������Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������ͣ�NiMH�е�M��ʾ���������Ͻ𣮸õ���ڳ��������ܷ�Ӧ�Ļ�ѧ����ʽ��Ni��OH��2+M=NiOOH+MH����NiMH��طŵ�����У������ĵ缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH-��
��7��һ����Ϊ��������Һ�е�����Ũ��С��1��10-5 mol/Lʱ�������Ѿ���ȫ���������ϱ������ݣ�����Fe��OH��2���ܶȻ�����1��10-13.6��mol/L��3���Ϳ��淴ӦFe2++2H2O=2H++Fe��OH��2 ��25��ʱ��ƽ�ⳣ��1��10-14.4��mol/L����
| A�� | ����Һ������pH=3������pH=11��NH3•H2O��Һ�������϶��� | |
| B�� | ����Һ�����ɵ����ʵ���Ũ�ȵ�NH3•H2O��������2��1������Ȼ�� | |
| C�� | ����Һ����ˮ�������������Ũ��һ������1.0��10-7mol•L-1 | |
| D�� | ����һ����NH3•H2O��c��NH4+�����ܴ��ڡ����ڻ�С��c��Cl-�� |
| A�� | ά��������ȻʳƷ�к����ḻ�����Լӹ����ʳƷ��ά���صĺ���Ҳ�� | |
| B�� | 2M+N=2P+2Q��2P+M=Q��M��NΪԭ�ϣ�QΪ������Ʒ�����ϡ���ѧ��Ӧ����ɫ������Ҫ�� | |
| C�� | ϡ��˫��ˮ��������ϴ�˿ڣ��Դﵽɱ����������Ŀ�� | |
| D�� | ������Si3N4��Al2O3�������½ṹ�մ���Ʒ |
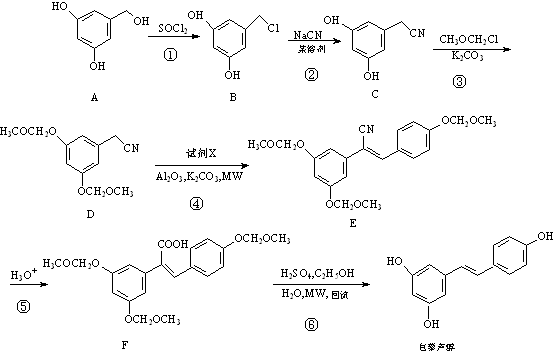
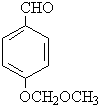 ��
��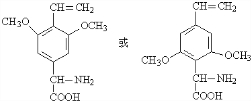 ����дһ�֣���
����дһ�֣���