��Ŀ����
8���������ʶ�Ӧ�ھ������͵�˵����Ϊ�������ǣ����������۵�1070�棬������ˮ��ˮ��Һ����
���۵�10.34�棬Һ̬�����磬ˮ��Һ����
��������CS2���۵�112.8�棬�е�444.6��
���۵�97.81�棬�������磬�ܶ�Ϊ0.97g•cm-3��
| A�� | �����Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| B�� | ��ԭ�Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| C�� | �����Ӿ���ڷ��Ӿ���۽�������ܽ������� | |
| D�� | ��ԭ�Ӿ�������Ӿ���۷��Ӿ���ܷ��Ӿ��� |
���� ���Ӿ��������������ӹ��ɣ����Ӽ����������ǿ�����нϸߵ��۵㡢�е㡢Ӳ�ȴ�����״̬��ˮ��Һ�ܵ�������ʣ�
��� �⣺���۵�1070�棬������ˮ��ˮ��Һ���磬�������Ӿ�����ص㣬�ʢ�Ϊ���Ӿ��壻
���۵�Ϊ10.31�棬�۵�ͣ����Ϸ��Ӿ�����ص㣬Һ̬�����磬������Һ̬ʱ��ֻ���ڷ��ӣ�û�����ӣ�ˮ��Һ�ܵ��磬����ˮ������ˮ���ӵ������£�����������ƶ������ӣ��ʢ�Ϊ���Ӿ��壻
��������CS2���۵�112.8�棬�е�444.6�棬���ڷ��Ӿ�����ص㣬�ʢ�Ϊ���Ӿ��壻
�ܽ������۵�Ϊ97.81�棬���������硢�ܶ�0.97g/cm3��Ϊ����������ص㣬�ʢ�Ϊ�������壻
��ѡA��
���� ������Ҫ���������Ӿ�����������ʣ�����ʱ���������Ӿ����۷е�������������ʼ��ɽ���ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
19�����й���Li��Na��K��Rb��Cs�����Ԫ�ص����ʱȽ��У�����ʵ����ȫ����ϵ��ǣ�������
| A�� | ԭ�Ӱ뾶�������� | B�� | ���Ӱ뾶�������� | ||
| C�� | ���ʵ��ܶ����������� | D�� | ��������ļ���������ǿ |
16������Ԫ�����ڱ��е�16��Ԫ�ص�˵����ȷ���ǣ�������
| A�� | ���������+5�� | B�� | �����Ϊ-2�� | ||
| C�� | ��Ԫ��λ�ڵڶ����� | D�� | ��Ԫ�����ڢ�A��Ԫ�� |
3�����ⶨ����C3H7OH��C2H5OC2H5��C6H12��ɵĻ������������������Ϊ8%����˻�����������������Ϊ��������
| A�� | 78% | B�� | 22% | C�� | 14% | D�� | 13% |
13�����и�ʽ����ʵ��ʾ���ʷ�����ɵ��ǣ�������
| A�� | SO2 | B�� | CaCl2 | C�� | SiO2 | D�� | Na2O2 |
6�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO��SiO2�����ʣ���ҵ�������̿���ȡMnSO4•H2O���������£�

��֪���ٲ��ֽ�����������ȫ����ʱ��pH���±�
���¶ȸ���27��ʱ��MnSO4������ܽ�����¶ȵ����߶����ͣ�
��1����������������MnO2ת��ΪMn2+�����ӷ���ʽΪSO2+MnO2�TMn2++SO42-��
��2����1�������м���H2O2��Ŀ���ǽ�Fe2+����ΪFe3+��
��3����1���������γ�����l����Ҫ�ɷ�ΪAl��OH��3��Fe��OH��3���ѧʽ������pH��5-6���ӵ��Լ�����ѡ��a��b���������Լ��������ĸ���� a��Cao b��MgO c��Al2O3 d����ˮ
��4����2�����ӣ���Ҫ�ǽ�Ca2+��Mg2+ת��Ϊ��Ӧ�����������ȥ��д��MnF2��ȥMg2+�����ӷ�Ӧ����ʽMnF2+Mg2+�TMn2++MgF2���÷�Ӧ��ƽ�ⳣ����ֵΪ7.2��107��
����֪��MnF2��Ksp=5.3��10-3��CaF2��Ksp=1.5��10-10��MgF2��Ksp=7.4��10-11����
��5�����á����ȹ��ˡ�������ԭ������Ϊ����MnSO4•H2O��ˮ�е��ܽ⣬�õ������ò�Ʒ��
��6��ȡ����MnSO4•H2O����ˮ�������Һ������pH���ָ���Һ�����ԣ�ԭ����Mn2++2H2O?Mn��OH��2+2H+�������ӷ���ʽ��ʾ��������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊc��SO42-����c��Mn2+����c��H+����c��OH-����

��֪���ٲ��ֽ�����������ȫ����ʱ��pH���±�
| ���������� | Fe3+ | Al3+ | Mn2+ | Mg2+ |
| ��ȫ����ʱ��PHֵ | 3.2 | 5.2 | 10.4 | 12.4 |
��1����������������MnO2ת��ΪMn2+�����ӷ���ʽΪSO2+MnO2�TMn2++SO42-��
��2����1�������м���H2O2��Ŀ���ǽ�Fe2+����ΪFe3+��
��3����1���������γ�����l����Ҫ�ɷ�ΪAl��OH��3��Fe��OH��3���ѧʽ������pH��5-6���ӵ��Լ�����ѡ��a��b���������Լ��������ĸ���� a��Cao b��MgO c��Al2O3 d����ˮ
��4����2�����ӣ���Ҫ�ǽ�Ca2+��Mg2+ת��Ϊ��Ӧ�����������ȥ��д��MnF2��ȥMg2+�����ӷ�Ӧ����ʽMnF2+Mg2+�TMn2++MgF2���÷�Ӧ��ƽ�ⳣ����ֵΪ7.2��107��
����֪��MnF2��Ksp=5.3��10-3��CaF2��Ksp=1.5��10-10��MgF2��Ksp=7.4��10-11����
��5�����á����ȹ��ˡ�������ԭ������Ϊ����MnSO4•H2O��ˮ�е��ܽ⣬�õ������ò�Ʒ��
��6��ȡ����MnSO4•H2O����ˮ�������Һ������pH���ָ���Һ�����ԣ�ԭ����Mn2++2H2O?Mn��OH��2+2H+�������ӷ���ʽ��ʾ��������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊc��SO42-����c��Mn2+����c��H+����c��OH-����
3������ʵ��װ�û���������ȷ���ܴﵽʵ��Ŀ���ǣ�������
| A�� | 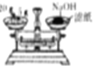 ����NaOH���� | B�� | 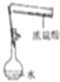 ����һ�����ʵ���Ũ��ϡ���� | ||
| C�� | 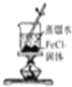 ��ȡ������Fe��OH��3���� | D�� | 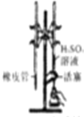 ��H2SO4����Һ�ζ�NaOH��Һ |
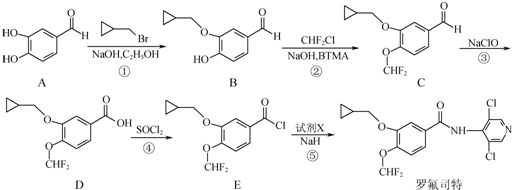
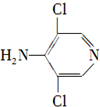 ��
��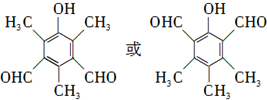 �ȣ�
�ȣ�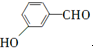 ��CH3CH2OHΪԭ���Ʊ�
��CH3CH2OHΪԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�