��Ŀ����
����Ŀ��ij�����п��ܺ���K+��NH4+��Mg2+��Fe3+��Ba2+��Cu2+��Cl-��SO42-��HCO3-�еļ��֣���д���пհף�
��1�����ù�������ˮ�����ɫ������Һ���ɴ�����ó��ù�����һ�������ڵ�������___��
��2����������ɫ������Һ���м�⣬ʵ�鲽�輰������ͼ��
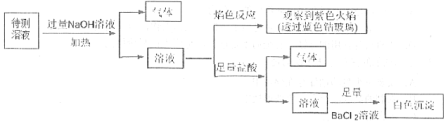
ͼ��ʵ���еõ�����������ֱ���___��___����ɫ�����ɷ���___ (�ѧʽ)��������������������������ӷ���ʽΪ___���ù����п��ܴ��ڵ�������___��
��3��ͨ�����ϼ�⣬����˵����ȷ����___
A.�ù����п��ܺ���(NH4)2SO4��KHCO3
B.�ù����п��ܺ���K2SO4��NH4HCO3��һ������KCl
C.�ù����п��ܺ���(NH4)2SO4��KCl��һ������KHCO3
���𰸡�![]() ��
��![]()
![]()
![]()
![]()
![]()
![]() A
A
��������
![]() �������NaOH��Һ�����ȣ����ɵ�����Ϊ
�������NaOH��Һ�����ȣ����ɵ�����Ϊ![]() ����ԭ��Һ�к���
����ԭ��Һ�к���![]() ����Ӧ��õ���Һ����ԭ��Һ��û��
����Ӧ��õ���Һ����ԭ��Һ��û��![]() ��
�� ![]() ��ɫ��Ӧ������ɫ�ܲ������������ɫ������
��ɫ��Ӧ������ɫ�ܲ������������ɫ������![]() ��
�� ![]() ��Һ�м����������ᣬ�������壬Ϊ
��Һ�м����������ᣬ�������壬Ϊ![]() ����ԭ��Һ�к���
����ԭ��Һ�к���![]() ��������������������������ӷ���ʽΪ
��������������������������ӷ���ʽΪ![]() ��
�� ![]() ����HCl����Һ�м���������
����HCl����Һ�м���������![]() ���а�ɫ
���а�ɫ![]() ���ɣ���ԭ��Һ�к���
���ɣ���ԭ��Һ�к���![]() �����ݹ��棬��ԭ��Һ����
�����ݹ��棬��ԭ��Һ����![]() ��
��
���ϣ�ԭ��Һ��һ�����У�![]() ��
��![]() ��
��![]() ��
��![]() ��һ��������
��һ��������![]() ��
��![]() ��
��![]() ��
��![]() �����ܺ���
�����ܺ���![]() ���ݴ˷������
���ݴ˷������
![]() ����Һ�ʻ�ɫ��
����Һ�ʻ�ɫ��![]() ����Һ����ɫ���ù�������ˮ�����ɫ������Һ��������Һ��û��
����Һ����ɫ���ù�������ˮ�����ɫ������Һ��������Һ��û��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
�� ![]() �������NaOH��Һ�����ȣ����ɵ�����Ϊ
�������NaOH��Һ�����ȣ����ɵ�����Ϊ![]() ����ԭ��Һ�к���
����ԭ��Һ�к���![]() ����Ӧ��õ���Һ����ԭ��Һ��û��
����Ӧ��õ���Һ����ԭ��Һ��û��![]() ��
�� ![]() ��ɫ��Ӧ������ɫ�ܲ������������ɫ������
��ɫ��Ӧ������ɫ�ܲ������������ɫ������![]() ��
�� ![]() ��Һ�м����������ᣬ�������壬Ϊ
��Һ�м����������ᣬ�������壬Ϊ![]() ����ԭ��Һ�к���
����ԭ��Һ�к���![]() ��������������������������ӷ���ʽΪ
��������������������������ӷ���ʽΪ![]() ��
�� ![]() ����HCl����Һ�м���������
����HCl����Һ�м���������![]() ���а�ɫ
���а�ɫ![]() ���ɣ���ԭ��Һ�к���
���ɣ���ԭ��Һ�к���![]() �����ݹ��棬��ԭ��Һ����
�����ݹ��棬��ԭ��Һ����![]() ��
��
���ϣ�ԭ��Һ��һ�����У�![]() ��
��![]() ��
��![]() ��
��![]() ��һ��������
��һ��������![]() ��
��![]() ��
��![]() ��
��![]() �����ܺ���
�����ܺ���![]() ������ʵ���еõ�����������ֱ���
������ʵ���еõ�����������ֱ���![]() ��
��![]() ����ɫ�����ɷ���
����ɫ�����ɷ���![]() ��������������������������ӷ���ʽΪ
��������������������������ӷ���ʽΪ![]() �������п��ܴ��ڵ�������
�������п��ܴ��ڵ�������![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
�� ![]() ����
����![]() �ķ�����֪����������ˮ������ɫ������Һ�У�һ�����У�
�ķ�����֪����������ˮ������ɫ������Һ�У�һ�����У�![]() ��
��![]() ��
��![]() ��
��![]() �����ܺ���
�����ܺ���![]() ��
��
A���ù����п��ܺ���![]() ��
��![]() ���������⣬A��ȷ��
���������⣬A��ȷ��
B�������п��ܴ��ڵ�������![]() ���ù����п��ܺ���
���ù����п��ܺ���![]() ��
��![]() �������ܺ���KCl��B����
�������ܺ���KCl��B����
C�����ɹ����������һ�����У�![]() ��
��![]() ��
��![]() ��
��![]() �����ܺ���
�����ܺ���![]() ���ù����п��ܺ���
���ù����п��ܺ���![]() ��KCl��
��KCl��![]() ��C����
��C����
�ʴ�Ϊ��A��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±���ij����Ӫ���ɷ֣�
Ӫ������ | ������ | ��֬ | ���� | ά������ | ̼��� |
����/ |
|
|
|
|
|
�����й�˵����ȷ���ǣ� ����
A.���ۺ���ά���������࣬�һ�Ϊͬ���칹��
B.ά������һ�ֻ���Ӫ������
C.���ۡ���֬�������ʶ���̼���⡢������Ԫ�����
D.���ۡ���֬�������ʶ�Ϊ�л��߷��ӻ�����