��Ŀ����
12����Ȼ������Ҫ�ɷ��Ǽ��飬������һ����Ҫ��ȼ�Ϻͻ�������ԭ�ϣ���1���Լ����ˮΪԭ�Ͽ���ȡ�״���
��CH4��g��+H2O��g��?CO��g��+3H2��g����H=+206.0kJ/mol
��CO��g��+2H2��g��?CH3OH��g����H=-129.0kJ/mol
��CH4��g��+H2O��g��?CH3OH��g��+H2��g���ġ�H=+77.0kJ/mol��
��2����ӦCH4��g��+H2O��g��?CO��g��+3H2��g����������ȡ�ϳɰ���ԭ����H2��
T��ʱ����1.0molCH4��2.0molH2O��g��ͨ��10L���ܱ���������5min�ﵽƽ��״̬��CH4��ת����Ϊ50%������H2��ʾ�ķ�Ӧ����v��H2��=0.03mol•L-1•min-1�����¶�ʱ��ƽ�ⳣ��K=2.25��10-2��
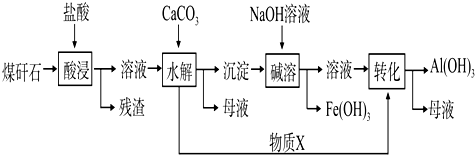
��3������������ͭ��һ�����͵�p�Ͱ뵼����ϣ����л��ԵĿ�Ѩ-���ӶԺ����õĴ����ԣ�������ص�����������������Ź㷺��Ӧ�ã���ҵ�Ͽ��õ�ⷨ��ȡ����Cu2O��2Cu+H2O$\frac{\underline{\;���\;}}{\;}$Cu2O+H2������ij��ѧ��ȤС���ͬѧ�������ͼ��ʾװ�ã�����ģ��ʵ�飮
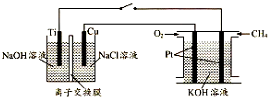
��װ�����ü���ȼ�յ�����ṩ���ܣ�ȼ�ϵ�ظ���ͨ��������Ǽ��飻װ����Cu�缫���������������ü��ĵ缫��ӦʽΪH2O+2Cu-2e-=Cu2O+2H+������Ӧ����������״����336mLCH4�������Ͽɵõ�8.64gCu2O��
���� ��1�����ݸ�˹���ɣ�Ŀ�귴Ӧ�ķ�Ӧ��Ϊ��-�ڣ�
��2��������ʽ��ʾ��������ʼ����ת������ƽ���������ݷ�Ӧ���ʼ��㹫ʽV=$\frac{��C}{��t}$����������ƽ�ⳣ������ʽ����÷�Ӧ��ƽ�ⳣ����
��3��ȼ�ϵ�ظ���ͨ��������Ǽ��飬ͨ����Ϊȼ�ϵ�ص�������������������ͭ�ǵ��ص�����������������Ӧ����Cu2O������ת�Ƶ����������������ͭ��������
��� �⣺��1����CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.0kJ•mol-1
��CO��g��+2H2��g���TCH3OH��g����H=-129.0kJ•mol-1
���ݸ�˹���ɣ���-�ڵã�CH4��g��+H2O��g��=CH3OH��g��+H2��g����H=+206.0kJ/mol-��-129.0kJ/mol��=+77.0 kJ/mol��
�ʴ�Ϊ��+77.0��
��2����1.0mol CH4��2.0mol H2O �� g ��ͨ���ݻ��̶�Ϊ10L�ķ�Ӧ�ң�����ת����Ϊ50%���ʲμӷ�Ӧ�ļ���Ϊ1mol��50%=0.5mol����
CH4 ��g��+H2O ��g��=CO ��g��+3H2 ��g��
��ʼ����mol����1.0 2.0 0 0
�仯����mol����0.5 0.5 0.5 1.5
ƽ������mol����0.5 1.5 0.5 1.5
����H2��ʾ�÷�Ӧ��ƽ�ⷴӦ����V��H2��=$\frac{\frac{1.5mol}{10L}}{5min}$=0.03 mol•L-1•min-1��
�ʴ�Ϊ��0.03 mol•L-1•min-1��
T��ʱ��ӦI��ƽ��Ũ��Ϊc��CH4��=0.05mol/L��c��H2O��=0.15mol/L��c��CO��=0.05mol/L��c��H2��=0.15mol/L��
���Ը��¶��·�Ӧ��ƽ�ⳣ��K=$\frac{c��CO����{c}^{3}��{H}_{2}��}{c��C{H}_{4}����c��{H}_{2}O��}$=$\frac{0.05��0.1{5}^{3}}{0.05��0.15}$=2.25��10-2��
�ʴ�Ϊ��2.25��10-2��
��3��ȼ�ϵ�ظ���ͨ��������Ǽ��飬ͨ����Ϊȼ�ϵ�ص�������������������ͭ�ǵ��ص�����������������Ӧ����Cu2O���缫��ӦʽΪ��H2O+2Cu-2e-=Cu2O+2H+��
CH4������8e-������4Cu2O
22.4��103 4��144
336mL m
����$\frac{22.4��1{0}^{3}}{336}$=$\frac{4��144}{m}$����֮��m=8.64g��
�ʴ�Ϊ�����飻������H2O+2Cu-2e-=Cu2O+2H+��8.64��
���� ����Ϊ�ۺ��⣬�����˸�˹���ɵ�Ӧ�á���Ӧ���ʡ�ƽ�ⳣ���ļ��㣬��Ϥ�Ȼ�ѧ����ʽ��д�ķ�������˹���ɼ��㷴Ӧ�ȵķ�������ȷ�绯ѧ�ķ�Ӧԭ���ǽ���Ĺؼ�����Ŀ�ѶȽϴ�

 ��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������
��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������| A�� | �û�����Ļ�ѧʽ��Y4Ba4Cu3O12 | B�� | �û�����Ļ�ѧʽ��YBaCu3O6 | ||
| C�� | �û�����Ļ�ѧʽ��Y2BaCu3O6 | D�� | �û�����Ļ�ѧʽ��YBa2Cu3O7 |
| A�� | ͬ����Ԫ�ص�ԭ�Ӱ뾶�Ԣ�A���Ϊ��С | |
| B�� | �����ڱ�������Ԫ�صĵ���ȫ�������� | |
| C�� | ��A����A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ����ʧȥ���� | |
| D�� | ��������Ԫ�ص�ԭ���γɵ�ԭ������ʱ�������������������������� |

�����ȡ���ָ���뺬����Ԫ�ص����ʣ������ʿ����ǣ�������
| A�� | ���� | B�� | ClO2 | C�� | NaCl | D�� | NaClO3 |
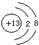 ������˵���в���ȷ���ǣ�������
������˵���в���ȷ���ǣ�������| A�� | ��Ԫ��ԭ�ӵ�ԭ�Ӻ�����2�����Ӳ� | |
| B�� | ��Ԫ����һ�ֽ���Ԫ�� | |
| C�� | �������������� | |
| D�� | �����Ӿ����ȶ��ṹ |
HX��aq��?X-��aq��+H+��aq����H��0 K=10-a
X-��aq��+H2O?HX��aq��+OH-��aq����H��0 k=10-b
�����й�Ũ�Ⱦ�Ϊ0.1mol•L-1HX��Һ��NaX��Һ��������ȷ���ǣ�������
| A�� | �ֱ������Һ��ʱ��K��������ҺpH����С | |
| B�� | �����·ֱ�ϡ������Һʱ��K�����䡢��ҺpH������ | |
| C�� | 25��ʱa+b=14 | |
| D�� | 25��ʱ����Һ�������pH=8����Һ�У�c��X-����c��Na+�� |
| A�� | �뵼������黯�� | B�� | ���մɲ�������п | ||
| C�� | ������������Ͻ� | D�� | ��������K3C60 |