��Ŀ����
7��ij��ҽҩ�м���ĽṹʽΪ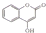 ���������Ʊ�����Ѫҩ����ͨ���������̺ϳɣ�
���������Ʊ�����Ѫҩ����ͨ���������̺ϳɣ�
��֪��F���G�൱�ڴ�F������ȥ��1��X���ӣ���ش�
��1�����й���G��˵����ȷ����ab��
a��������ˮ��Ӧ
b����������������Һ��Ӧ
c��1molG����ܺ�3mol������Ӧ
d������FeC1��3��Һ��Ӧ����ɫ
��2��B��C�ķ�Ӧ�����ǣ�
��3��д�������йط�Ӧ�Ļ�ѧ����ʽ����A������������ͭ����Һ����CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O��
��D��E��
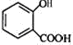 +CH3OH$?_{��}^{Ũ����}$
+CH3OH$?_{��}^{Ũ����}$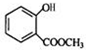 +H2O��
+H2O����4��F��������6�ֻ�ѧ������ͬ����ԭ�ӣ�������X�Ľṹ��ʽΪCH3OH��
��5��д����D��Ϊͬ���칹���Һ����������ǻ����������л����ͬ���칹��Ľṹ��ʽ
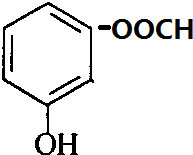 ��
��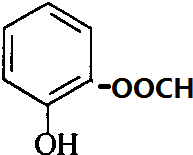 ��
��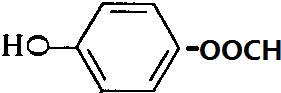 ��
��
���� A��������ΪCH3COOH����AΪCH3CHO����F��C��֪EΪ ������D�ķ���ʽC7H6O3�Լ�E�Ľṹ��ʽ�����ƶ�DΪ
������D�ķ���ʽC7H6O3�Լ�E�Ľṹ��ʽ�����ƶ�DΪ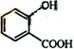 ������״���Ӧ����E����XΪCH3OH���ݴ˴��⣮
������״���Ӧ����E����XΪCH3OH���ݴ˴��⣮
��� �⣺A��������ΪCH3COOH����AΪCH3CHO����F��C��֪EΪ ������D�ķ���ʽC7H6O3�Լ�E�Ľṹ��ʽ�����ƶ�DΪ
������D�ķ���ʽC7H6O3�Լ�E�Ľṹ��ʽ�����ƶ�DΪ ������״���Ӧ����E����XΪCH3OH��
������״���Ӧ����E����XΪCH3OH��
��1������G�Ľṹ��ʽ��֪��G�к��еĺ���������Ϊ�ǻ���������̼̼˫���������ܹ�����ˮ�����ӳɷ�Ӧ��������NaOH��Һ�з���ˮ�ⷴӦ��1molG����ܹ���4mol�����ӳɣ�G��û�з��ǻ����������Ȼ�����Һ������ɫ��Ӧ��
�ʴ�Ϊ��ab��
��2��B��C���Ȼ����ǻ�����ԭ��ȡ������ȡ����Ӧ��
�ʴ�Ϊ��ȡ����Ӧ��
��3����AΪCH3CHO��������������ͭ����Һ�ڼ��������·�����Ӧ�Ļ�ѧ����ʽΪCH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O��
�ʴ�Ϊ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O��
��D��E�Ļ�ѧ����ʽΪ +CH3OH$?_{��}^{Ũ����}$
+CH3OH$?_{��}^{Ũ����}$ +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ�� +CH3OH$?_{��}^{Ũ����}$
+CH3OH$?_{��}^{Ũ����}$ +H2O��
+H2O��
��4������F�Ľṹ��ʽ��֪��F�������²��Գƣ�����������Ƿ��ǻ��������γɵ���������������DZ�����ͼ״��γɵ�������������4�ֻ�ѧ������ͬ����ԭ�ӣ���������ѧ��������ͬ������F��������6�ֻ�ѧ������ͬ����ԭ�ӣ���ԭ���غ��F��G�Ľṹ��ʽ��֪����F������ȥ��1��X����Ϊ�״����ʴ�Ϊ��6��CH3OH��
��5��DΪ �����б�����������ͬ���칹��ӦΪ���ᱽ�������Һ��з��ǻ������ڡ��䡢��3�֣�������
�����б�����������ͬ���칹��ӦΪ���ᱽ�������Һ��з��ǻ������ڡ��䡢��3�֣������� ��
�� ��
�� ��
��
��
�ʴ�Ϊ�� ��
�� ��
�� ��
��
���� ���⿼���л���ĺϳɣ���Ŀ�Ѷ��еȣ�����ע����A��EΪ�ƶϸ����ͻ�ƿڽ��н��ע������ŵı仯�����������Ϣ��

| A�� | �ǽ�����X��Y��Z | |
| B�� | �����������H3ZO4��H2YO4��HXO4 | |
| C�� | Ԫ�صĸ����ϼ۵ľ���ֵ��X��Y��Z��С | |
| D�� | ԭ�Ӱ뾶��X��Y��Z |
��1������ˮ��Ӧ��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2��g����H
��֪����3Fe��s��+2O2��g��?Fe3O4��s����H1=-1118.4kJ/mol
��2H2��g��+O2��g��?2H2O��g����H2=-483.8kJ/mol
��2H2��g��+O2��g��?2H2O��l����H3=-571.8kJ/mol
���H=-150.8KJ/mol������������һλС������
��2������t��ʱ���÷�Ӧ�ﵽƽ��״̬��û������ƽ����Է�������Ϊ$\frac{22}{3}$����Ӧ��ƽ�ⳣ��K=16��
������˵����ȷ����B������ĸ��ţ�
A��������ѹǿ�㶨����Ӧ�ﵽƽ��״̬
B�����������ܶȺ㶨����Ӧ�ﵽƽ��״̬
C�����º���ƽ���������H2���ٴ�ƽ���H2O��g���������������
D������Fe3O4�������H2O��ת����
��3�������ͷ����ں��ݾ��ȵ�װ���У����±�������ʼ���ʣ���ʼʱ��ƽ���ĸ����ʵ������±���
| Fe | H2O��g�� | Fe3O4 | H2 | |
| ��ʼ/mol | 3.0 | 4.0 | 0 | 0 |
| ƽ��/mol | m | n | p | Q |
| Fe | H2O��g�� | F3O4 | H2 | |
| A/mol | 3.0 | 4.0 | 0 | 0 |
| B/mol | 0 | 0 | 1.0 | 4.0 |
| C/mol | m | n | p | Q |
���������淴Ӧ��һ�δﵽƽ��״̬��������װ����H2����������ɴ�С��˳�����У�B��C��A����A��B��C��ʾ��
������Һ����һ���������Σ�Ϊ��ˮ��ϵ�����л������ӡ�Al2Cl7-��AlCl4-��ɵ�����Һ�������Һʱ�����ڸ���Ʒ�ϵ������
��1������ƷӦ�ӵ�Դ�ĸ�������֪��ƹ����в����������������л������Ӳ�����缫��Ӧ�������缫��ӦʽΪ4Al2Cl7-+3e-=Al+7AlCl4-��
��2��������AlCl3ˮ��Һ�����Һ����һ��ʱ���������AlO2-���ӣ������ܻ��ܣ�
| A�� | Aԭ�ӵ�������������Bԭ�ӵ������������� | |
| B�� | Aԭ�ӵĵ��Ӳ�����Bԭ�ӵĵ��Ӳ����� | |
| C�� | 1mol A�������û�H+���ɵ�H2��1mol B�������û�H+���ɵ�H2�� | |
| D�� | ����ʱ��A�ܴ�ˮ���û����⣬��B���� |
| A�� | 0.11mol | B�� | 0.22mol | C�� | 0.16mol | D�� | 0.1mol |