��Ŀ����
2�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������Ӳ�����ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ���壬e�������ֻ��1�����ӣ��ڲ���Ӷ��������ش��������⣺��1��b��c��d�е�һ�������ɴ�С��˳�����N��O��C����Ԫ�ط��ţ���e����Χ�����Ų�ʽΪ3d104s1��
��2��a��b�γ�һ����Է�������Ϊ32��һ�ֻ�����÷��ӵ�����ԭ�ӵ��ӻ���ʽΪsp3��
a��c�γ�һ����Է�������Ϊ34��һ�ֻ�����÷��ӵ�����ԭ�ӵ��ӻ���ʽΪsp3��
��3��b��c��d��e�γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ���û�������������ΪSO42-��1mol������[e��bH3��4]2+�к��ЦҼ���ĿΪ16mol��16NA�����������д��ڵĻ�ѧ��������ADG��
A����λ�� B�������� C�����Ӽ� D�����Թ��ۼ�
E���Ǽ��Թ��ۼ� F����� G���Ҽ� H���м�
��4����e���ʵķ�ĩ����bH3��Ũ��Һ�У�ͨ��c2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
���� ���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ����aΪHԪ�أ�
c������������Ϊ���ڲ��������3����ԭ��������������8������c��OԪ�أ�d��cͬ�壬��d��SԪ�أ�
b�ļ۵��Ӳ��е�δ�ɶԵ�����3������ԭ������С��c����b��NԪ�أ�
e�������ֻ��һ�����ӣ��ڲ���Ӷ�����������ԭ����������S����e��������Ų�ʽΪ��1s22s22p63s23p63d104s1����e��CuԪ�أ��ٽ��ԭ�ӽṹ�����ʽṹ��Ԫ�������ɽ��
��� �⣺���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ����aΪHԪ�أ�c������������Ϊ���ڲ��������3����ԭ��������������8������c��OԪ�أ�d��cͬ�壬��d��SԪ�أ�b�ļ۵��Ӳ��е�δ�ɶԵ�����3������ԭ������С��c����b��NԪ�أ�e�������ֻ��һ�����ӣ��ڲ���Ӷ�����������ԭ����������S����e��������Ų�ʽΪ��1s22s22p63s23p63d104s1����e��CuԪ�أ�
��1��b��c��d�ֱ���N��O��SԪ���У�Ԫ�صķǽ�����Խǿ�����һ������Խ��ͬһ����Ԫ���У���һ����������ԭ��������������������ƣ���Nԭ�ӵ�2p������ڰ���״̬�����һ�����ܴ���OԪ�أ�����N��O��S�е�һ�����ܴ�СΪ��N��O��C��
eΪͭԪ�أ����ļ۲�����Ų�ʽΪ��3d104s1��
�ʴ�Ϊ��N��O��C��3d104s1��
��2��a��b�γ�һ����Է�������Ϊ28��һ�ֻ�����û�����Ϊ��N2H4��N2H4��Nԭ������һ��Nԭ���γ�һ�����ۼ�����������ԭ���γ��������ۼ������γ��������ۼ�������һ�Թ¶Ե���ռ��һ���ӻ�����������γ���4���ӻ�������ӻ�����Ϊsp3��
a��c�γ�һ����Է�������Ϊ34��һ�ֻ�����û�����Ϊ��H2O2��H2O���ӵ�����ԭ��Oԭ������һ��Oԭ���γ�1�����ۼ�����1��Hԭ���γ�1����������ܹ��γ����������ۼ�������2�Թµ��Ӷ�ռ�������ӻ�������ܹ��γ���4���ӻ�������ӻ�����Ϊsp3��
�ʴ�Ϊ��sp3��sp3��
��3��a��b��d��e�ֱ���H��N��S��CuԪ�أ�����Ԫ���γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ�������ӳ�������ṹ��˵���������Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�ΪSO42-��
[e��bH3��4]2+Ϊ[Cu��NH3��4]2+��1mol�����к���3mol�Ҽ����ܹ����ЦҼ������ʵ���Ϊ��3mol��4=12mol��1mol��������к���4mol��λ�������Ժ��еĦҼ������ʵ���Ϊ��12mol+4mol=16mol������16NA�������������к�����λ�����Ҽ��ͼ��Լ���
�ʴ�Ϊ��SO42-��16NA��16mol��ADG��
��4����Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ��˵����Ӧ������[Cu��NH3��4]2+���÷�Ӧ�����ӷ���ʽΪ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
�ʴ�Ϊ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ������ƶϸ�Ԫ������Ϊ���ؼ��������漰�縺�Դ�С�Ƚϡ������Ų�ʽ����ѧ�����͵��жϡ��ӻ������жϵ�֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ�����Ӧ����ѧ֪ʶ��������

| A�� | ͨ��״���£�NO2����ɫ������ | |
| B�� | SO2��Ư��Ʒ������ʣ�˵��SO2�������� | |
| C�� | ����ʱ����������Ũ���������Ӧ | |
| D�� | �������Ⱦ���ǿ�����ԣ�����������ˮ��ɱ������ |
| A�� | ��������Һ������ȩ�е�ȩ����CH3CHO+2[Ag��NH3��2]++2OH-$\stackrel{ˮԡ����}{��}$CH3COO-+NH4++3NH3+2Ag��+H2O | |
| B�� | ��������Һ�еμӹ�����ˮ��Ag++NH3•H2O�TAgOH��+NH${\;}_{4}^{+}$ | |
| C�� | ��NaHSO4��Ba��OH��2��Һ��������ԣ�H++SO42-+Ba2++2OH-�TBaSO4��+H2O | |
| D�� | ��ˮͨ������SO2��SO2+2NH3•H2O=2NH4++SO32-+H2O |
| A�� |  ����������FeCl3 ��Һ��ô�FeCl3 ���� | |
| B�� |  ���������ſ������ռ�CO2��NO ������ | |
| C�� |  ����ʵ���������϶���һ��ͭ | |
| D�� |  ���Գ�ȥCl2�л��е�HCl |
��1��C��s��+O2��g���TCO2��g����H1=-393.5kJ•mol-1
��2��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H2=-285.8kJ•mol-1
��3��CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H3=-870.3kJ•mol-1
�������Ϸ���ʽ���Լ����2C��s��+2H2��g��+O2��g���TCH3COOH��l���ķ�Ӧ��Ϊ��������
| A�� | +244.1 kJ•mol-1 | B�� | -488.3 kJ•mol-1 | C�� | -996.6 kJ•mol-1 | D�� | +996.6 kJ•mol-1 |
 �����ᣨHN02���ڹ�ҵ�������л��ϳɣ��Ⱦ����������־��л�ԭ�ԣ����������ԱȻ�ԭ��ͻ���ö࣮�ش��������⣺
�����ᣨHN02���ڹ�ҵ�������л��ϳɣ��Ⱦ����������־��л�ԭ�ԣ����������ԱȻ�ԭ��ͻ���ö࣮�ش��������⣺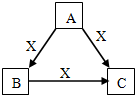 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ����ͼ��������������������ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ����ͼ��������������������ͬ����ش�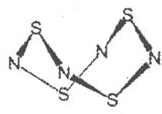
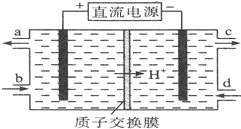
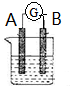 ��ͼ��ʾΪԭ���װ��ʾ��ͼ��
��ͼ��ʾΪԭ���װ��ʾ��ͼ��