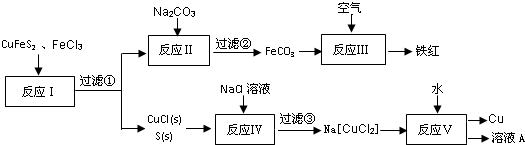
��Ŀ����
11��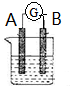 ��ͼ��ʾΪԭ���װ��ʾ��ͼ��
��ͼ��ʾΪԭ���װ��ʾ��ͼ����1����AΪZn��BΪʯī�����������ҺΪϡ���ᣬ��A�缫Ϊ�������������������д�������ĵ缫��Ӧʽ2H++2e-=H2����
��2����AΪCu��BΪFe���������ҺΪCuCl2��Һ����CuƬ��Ϊ�������������������д�������ĵ缫��ӦʽCu2++2e-=Cu������������1mol����ͨ��ʱ�������ϻ�������Ƭ28g��
��3����AΪFe��BΪʯī�����������ҺΪ�Ȼ�����Һ����һ���������ܲ�������Һ�й���������ڵ�����B����A������ĸA��B����д�������ĵ缫��Ӧʽ��O2+H2O+4e-=4OH-��
��4����A��B��Ϊ��������pt��Ƭ���������ҺΪϡ���ᣬ�ֱ��A��B����ͨ��H2��O2 ���õ�ؼ�Ϊȼ�ϵ�أ�ͨ��O2��һ��Ϊ�õ�ص��������������������д�������ĵ缫��ӦʽO2+4H++4e-=2H2O��
��5����AΪMg��BΪAl���������ҺΪNaOH��Һ����A�缫Ϊ�������������������д�������缫��Ӧʽ2Al-6e-+8OH-=2AlO2-+4H2O������NaOH��Һ����ϡ���ᣬ���ʱA�缫Ϊ�����������������������ܷ�Ӧ�����ӷ���ʽΪMg+2H+=Mg2++H2����
��6����AB��Ϊ��������Pt��Ƭ���������ҺΪKOH��Һ���ֱ��AB����ͨ��CH4��O2 ���õ�ؼ�Ϊ����ȼ�ϵ�أ�ͨ��CH4��һ��Ϊ�õ�صĸ������������������д���õ缫�ĵ缫��ӦʽCH4+10OH--8e-=CO32-+7H2O������ܷ�Ӧ�����ӷ���ʽΪCH4+2O2+2OH-�TCO32-+3H2O��
���� ��1����ԭ����У��������ҺΪϡ����ʱ��п��ʧ������������CuΪ�����������������ӷŵ�����������
��2������ͭ���ã���Ϊ������ͭΪ������
��3����AΪFe��BΪʯī�����������ҺΪ�Ȼ�����Һ����һ���������ܲ�������Һ�й������������������ʴ����Ϊ������ʯīΪ������
��4����A��B��Ϊ��������pt��Ƭ���������ҺΪϡ���ᣬ�ֱ��A��B����ͨ��H2��O2 ���õ�ؼ�Ϊȼ�ϵ�أ�����Ϊ������������Ϊ������
��5����AΪMg��BΪAl���������ҺΪNaOH��Һ����Ϊ������þΪ����������NaOH��Һ����ϡ���ᣬþΪ������
��6����AB��Ϊ��������Pt��Ƭ���������ҺΪKOH��Һ���ֱ��AB����ͨ��CH4��O2 ���õ�ؼ�Ϊ����ȼ�ϵ�أ�ͨ��CH4��һ��Ϊ��������������
��� �⣺��1����ԭ����У��������ҺΪϡ����ʱ��п��ʧ������������CuΪ�����������������ӷŵ������������缫��ӦΪ2H++2e-=H2����
�ʴ�Ϊ������2H++2e-=H2����
��2������ͭ���ã���Ϊ������ͭΪ�����������ĵ缫��ӦʽΪCu2++2e-=Cu������ΪFe-2e-=Fe2+������������1mol����ͨ��ʱ�������ϻ�������Ƭ0.5mol������Ϊ0.5mol��56g/mol=28g��
�ʴ�Ϊ������Cu2++2e-=Cu��28g��
��3����AΪFe��BΪʯī�����������ҺΪ�Ȼ�����Һ����һ���������ܲ�������Һ�й������������������ʴ����Ϊ������ʯīΪ������������ʯī��������������������������ԭ��Ӧ���缫����ʽΪO2+H2O+4e-=4OH-���ʴ�Ϊ��B��A��O2+H2O+4e-=4OH-��
��4����A��B��Ϊ��������pt��Ƭ���������ҺΪϡ���ᣬ�ֱ��A��B����ͨ��H2��O2 ���õ�ؼ�Ϊȼ�ϵ�أ�����Ϊ������������Ϊ������������ӦΪO2+4H++4e-=2H2O���ʴ�Ϊ������O2+4H++4e-=2H2O��
��5����AΪMg��BΪAl���������ҺΪNaOH��Һ����Ϊ������þΪ�����������缫����ʽΪ2Al-6e-+8OH-=2AlO2-+4H2O������NaOH��Һ����ϡ���ᣬþΪ����������ܷ�Ӧ�����ӷ���ʽΪMg+2H+=Mg2++H2�����ʴ�Ϊ������2Al-6e-+8OH-=2AlO2-+4H2O������Mg+2H+=Mg2++H2����
��6����AB��Ϊ��������Pt��Ƭ���������ҺΪKOH��Һ���ֱ��AB����ͨ��CH4��O2 ���õ�ؼ�Ϊ����ȼ�ϵ�أ�ͨ��CH4��һ��Ϊ���������������缫����ʽΪCH4+10OH--8e-=CO32-+7H2O������ܷ�Ӧ�����ӷ���ʽΪCH4+2O2+2OH-�TCO32-+3H2O���ʴ�Ϊ������CH4+10OH--8e-=CO32-+7H2O��CH4+2O2+2OH-�TCO32-+3H2O��
���� �����ۺϿ�����ԭ���ԭ����Ϊ��Ƶ���㣬��ȷԭ������������жϷ����ǽⱾ��ؼ���ԭ���ԭ���Ǹ��л�ѧ���ص�Ҳ���ѵ㣬Ҫע������ԭ���ԭ�������ձ��ʣ���ȷ��д�缫��Ӧ����ʽ����Ŀ�Ѷ��еȣ�

| A�� | �ñ���ȡ��ˮ�еĵⵥ�� | |
| B�� | �÷�Һ©������ˮ�����������Ļ���� | |
| C�� | �����Ը��������Һ��ȥ�����л��е�������ϩ���Ի�ô����ļ��� | |
| D�� | �����£�����Ƭ����Ũ������Ƭ�̣�ȡ��ϴ�����ٽ���CuSO4��Һ�У���������֤������Ũ����ۻ� |
| A�� | ���ͼױ�������Ũ���ᷢ�������ϵ�ȡ����Ӧ | |
| B�� | ���ͼױ�����ʹ���Ը��������Һ��ɫ | |
| C�� | ���ͼ�Ȳ����ʹ������Ȼ�̼��Һ������ѧ��Ӧ����ɫ | |
| D�� | ����������±�ص��ʷ���ȡ����Ӧ����Ҫ�������� |

 ��
�� ��
��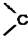 =O$��_{H+}^{ROH}$
=O$��_{H+}^{ROH}$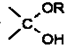 $��_{H+}^{ROH}$
$��_{H+}^{ROH}$ д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
д������ϩ���״�Ϊ�л�ԭ���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��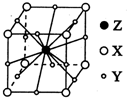 X��Y��Z��WΪ������Ԫ����ԭ��������������X��һ����̬�⻯��m���������ӻ��и�������Ҹ÷�������ԭ����һ��ֱ���ϣ�Y2-��M�ܲ��ԭ�ӹ��Ϊȫ��״̬��Z�ĵ����������ȷ�Ӧ����ȼ���� W��X�γɵĻ�������һ�ֳ������л��ܼ�n��AΪ��������Ԫ�أ����̬ԭ�Ӻ���6��δ�ɶԵ��ӣ�
X��Y��Z��WΪ������Ԫ����ԭ��������������X��һ����̬�⻯��m���������ӻ��и�������Ҹ÷�������ԭ����һ��ֱ���ϣ�Y2-��M�ܲ��ԭ�ӹ��Ϊȫ��״̬��Z�ĵ����������ȷ�Ӧ����ȼ���� W��X�γɵĻ�������һ�ֳ������л��ܼ�n��AΪ��������Ԫ�أ����̬ԭ�Ӻ���6��δ�ɶԵ��ӣ�