��Ŀ����
12�� ��1����̬��ԭ�ӵļ۵����Ų�ʽΪ3d54s1��
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ3d54s1����2��CrO2Cl2��NaClO�������������������������Ȼ������Ʊ�CrO2Cl2�ķ�ӦΪ��K2Cr2O7+3CCl4�T2KCl+2CrO2Cl2+3COCl2����������Ӧʽ�зǽ���Ԫ�ص縺���ɴ�С��˳����O��Cl��C����Ԫ�ط��ű�ʾ����
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е���-19.5�棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65�棩���״��ķе����Ը��ڼ�ȩ����Ҫԭ���Ǽ״����Ӽ�������������ȩ���Ӽ䲻���������
��4��˫�谷�ṹ��ʽ��ͼ1��ʾ��
��˫�谷�����ЦҼ��ͦм���Ŀ֮��Ϊ3��1��
��˫�谷����̼ԭ�ӵ��ӻ�����Ϊsp��sp2��
��5����Ԫ�����γɶ�������������[Ni��CN��4]2-�в����е���������AE����ѡ���ţ���
A�����Ӽ� B����λ�� C���Ҽ� D���м� E�����
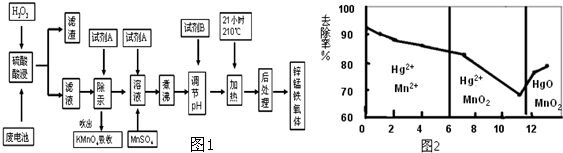
��6��������Ա�о�����������Ϊ�缫�Ĺ⻯ѧ��أ������������������ȵ缫��ʹˮ�ֽ������������֪�����Ⱦ����ṹ��ͼ2��ʾ�����仯ѧʽΪSrTiO3��
���� ��1��CrԪ��ԭ�Ӻ��������Ϊ24�����ݺ�������Ų����ɼ����ع���������д��
��2����Ӧʽ�зǽ���Ԫ�������֣�O��C��Cl����ϻ�����CCl4��NaClO��Ԫ�ػ��ϼ��жϣ�
��3�����ݼ״����Ӽ����������
��4���ٵ���Ϊ�Ҽ��ͣ�˫������1���Ҽ���1���м�������������1���Ҽ���2���м���
��ԭ���ӻ������Ŀ=�Ҽ���+�µ��Ӷԣ���Ͻṹ��ʽȷ��Cԭ���ӻ������Ŀ������ȷ�����ӻ���ʽ��
��5��̼����Ϊ��������������1���Ҽ���2���м���������Ϊ��λ����
��6�����ݾ�̯�����㾧����Sr��Ti��Oԭ����Ŀ������ȷ����ѧʽ��
��� �⣺��1��CrԪ��ԭ�Ӻ��������Ϊ24�����ݺ�������Ų����ɼ����ع�����������֪�۵����Ų�ʽΪ��3d54s1��
�ʴ�Ϊ��3d54s1��
��2����Ӧʽ�зǽ���Ԫ�������֣�O��C��Cl��CCl4��C���������ϼۡ�Cl���ָ����ϼۣ�NaClO��ClΪ+1�ۣ�OΪ-2�ۣ��縺��Խ�Լ��ϵ���������Խ��Ԫ�������ʱ��Ԫ�ر��ָ��ۣ��ʵ縺�ԣ�O��Cl��C��
�ʴ�Ϊ��O��Cl��C��
��3���״����Ӽ�������������ȩ���Ӽ䲻����������ʼ״��ķе�ϸߣ�
�ʴ�Ϊ���״����Ӽ�������������ȩ���Ӽ䲻���������
��4���ٵ���Ϊ�Ҽ��ͣ�˫������1���Ҽ���1���м�������������1���Ҽ���2���м���˫�谷������9���Ҽ�����3���м���˫�谷�����ЦҼ��ͦм���Ŀ֮��Ϊ9��3=3��1���ʴ�Ϊ��3��1��
��-C��N��Cԭ���γ�2���Ҽ������Ӱ�����Cԭ�ӳ�3���Ҽ�����û�йµ��Ӷԣ�Cԭ���ӻ������ֱ�Ϊsp��sp2���ʴ�Ϊ��sp��sp2��
��5��������[Ni��CN��4]2-����1���Ҽ���2���м���������Ϊ��λ�������������Ӽ����������ѡ��AE��
��6���ɾ����ṹ��֪��������Srԭ����ĿΪ8��$\frac{1}{8}$=1��Tiԭ����ĿΪ1����ԭ���γ���������ṹ��Oԭ����ĿΪ6��$\frac{1}{2}$=3���ʸ����ʻ�ѧʽΪ��SrTiO3���ʴ�Ϊ��SrTiO3��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����縺�ԡ��������ѧ�����ӻ�������������㣬��2����ע�����Ԫ�ػ���ʱԪ�ػ��ϼ��ж�O��ClԪ�ص縺�ԣ���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | ��NaCl��NaBr��NaI�����Һ��ͨ��F2��I-��Br-��Cl- | |
| B�� | ��NaCl��NaI��Na2S�����Һ�еμ�AgNO3��Һ��S2-��I-��Cl- | |
| C�� | ��FeCl3��CuCl2��HCl�����Һ�м���Zn�ۣ�Cu2+��Fe3+��H+ | |
| D�� | ��NaAlO2��Na2CO2��NaOH�����Һ�еμ�ϡ���AlO2-��CO32-��HCO3-��OH- |
| �ɷ� | Ũ��/��mol•L-1�� | �������� |
| HCl FeCl2 FeCl3 | -- 1.920 0.071 | 5.00% 8.94% 0.33% |
��2������ʯī���缫���������ϴ��Һʱ����ʼ�Σ������������������ɣ���ʱ��������йص������缫��ӦʽΪ2Cl--2e-=Cl2������������ϴ��Һ�м���KOH��Һ�кͺ��ں��ʵĵ�ѹ�µ�⣬��������������������������ɸ�����أ�K2FeO4����
��3������������ϴ��Һ��������ʯ����Ҫ�ɷ�ΪAl2O3��Fe2O3��SiO2���Լ����ƵĹ��ᣨ����ᣩ���Ʊ��۹����Ȼ����������������PAFSC�������巽����ͼ1��
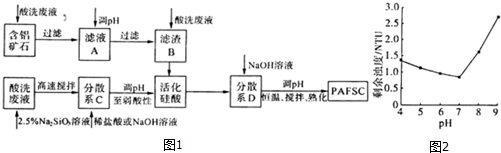
���ʵ�������ҺA��pH��Al3+��Fe3+ת��Ϊ������ԭ���ǵ�����ҺpH����Һ��[OH-]���Ӷ�ʹAl��OH��3��Fe��OH��3��Qc������Ksp�����������ҺpH����Һ��[OH-]���Ӷ�ʹAl��OH��3��Fe��OH��3�ij����ܽ�ƽ������������ƶ��������ó����ܽ�ƽ������۽��ͣ���
��PAFSC����������ˮ�Ĺ����У�Al3+���뷴Ӧ�����ӷ���ʽΪAl3++3H2O=Al��OH��3�����壩+3H+��
��25��ʱ��PAFSC�ij���Ч������ҺpH�ı仯��ͼ2��ʾ��ͼ�е�NTUΪ�Ƕȵ�λ������������pH��Χ�У�PAFSC����Ч����ѵ���b�������������ĸ����
a��4��5 b��5��7 c��7��8d��8��9
25��ʱ��pH��7����pH����PAFSC�ij���Ч�����Ա�ԭ���Ǽ�����ǿ��ʹ���巢���˾۳�����
| A�� | �٢ܢ� | B�� | �ڢۢܢݢ� | C�� | �ܢݢޢ� | D�� | ȫ�� |
| A�� | ������C��H��O�ĸ�����Ϊ1��2��3 | B�� | ������C��H�ĸ�����Ϊ1��2 | ||
| C�� | ���л������Է�������Ϊ14 | D�� | �÷����п϶������� |

| A�� | ƽ��ʱ�÷�Ӧ��ƽ�ⳣ��=$\frac{16}{3}��mol/L��^{-2}$ | |
| B�� | ƽ��ʱCO2��ת����Ϊ50% | |
| C�� | t2ʱ�̸ı�������Ǹ��¶� | |
| D�� | ��0-3minʱH2�ķ�Ӧ����Ϊ0.5mol•��L•min��-1 |