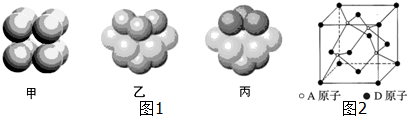
��Ŀ����
20������a-h8�ֶ�����Ԫ�أ�������Ԫ�����ڱ��е�λ��������ʾ���ݴ˻ش����⣺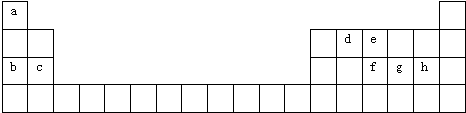
��1������Ԫ��ԭ�Ӽ������γ����Ӽ�����B����ѧʽΪNaCl�� �����γɹ��ۼ�����C����ѧʽΪCCl4��
A��c��f B��b��h C��d��h D��b��e
��2�����з���������ԭ�Ӷ����������8���ӽṹ����ACD
A��ea3 B��bhC��fh3 D��dh4
��3������a��b��g����Ԫ���γɵ��������ӻ����������һ������Է�������Ϊ120���û������ڻ�ʱ�ƻ��������Ӽ������뷽��ʽΪNaHSO4=Na++HSO4-������ˮʱ�ƻ��������Ӽ������ۼ������뷽��ʽΪNaHSO4=Na++H++SO42-������a��b��g����Ԫ���γɵĸ��������ӻ�������Ӧ�������д̼�����ζ�����壬��Ӧ�����ӷ���ʽΪH++HSO3-=SO2��+H2O����д�ڵ��뷽��ʽ��һ�У�
���� �����ڱ���λ��֪��aΪH��bΪNa��cΪMg��dΪC��eΪN��fΪP��gΪS��hΪCl��
��1�����õĽ�������õķǽ���֮���������γ����Ӽ����ǽ���Ԫ�ؼ�һ���γɹ��ۼ���
��2���������ƶϵó���ԭ���ж��仯�������������������ɣ�
��3����H��Na��S��O����Ԫ�ع��ɵ������Ӧ�����ӻ�����²���NaHSO3��NaHSO4���ݴ��ж���д���뷽��ʽ�����ӷ�Ӧ����ʽ���ɣ�
��� �⣺��1���ɶ�Ӧ���ڱ���λ��֪��aΪH��bΪNa��cΪMg��dΪC��eΪN��fΪP��gΪS��hΪCl��8��Ԫ��������õĽ�����������õķǽ�����֮���������γ����Ӽ����ǽ���Ԫ�ؼ�һ���γɹ��ۼ����ʴ�Ϊ��B��NaCl��C��CCl4��
��2����ѡ���Ӧ�ķ��ӷֱ�ΪNCl3��NaCl��PCl3��CCl4��NaClΪ���ӻ����������ԭ�ӣ��ʲ��������⣬����NCl3��PCl3�У�������ԭ�Ӻ�������������Ϊ5��������ԭ���γɹ��ۼ�ʱ������NCl3��PCl3�еĵ�����ԭ�ӡ���ԭ������㶼�ﵽ8���ӽṹ��ͬ����CCl4��������⣬��ѡA��C��D��
��3������H��Na��S��O����Ԫ�ع��ɵ������Ӧ�����ӻ�����²���NaHSO3��NaHSO4������ʽ��Ϊ120��ΪNaHSO4���ۻ�ʱ�ƻ��������Ӽ������뷽��ʽΪ��NaHSO4�TNa++HSO4-������ˮʱ�ƻ��������Ӽ����ۼ������뷽��ʽΪ��NaHSO4�TNa++H++SO42-������H��Na��S��O����Ԫ�ع��ɵ������Ӧ�����ӻ�����²���NaHSO3��NaHSO4�����Ӧ���ɶ����������壬���ӷ�Ӧ����ʽΪ��H++HSO3-=SO2��+H2O��
�ʴ�Ϊ�����Ӽ���NaHSO4=Na++HSO4-�����Ӽ����ۼ���NaHSO4=Na++H++SO42-��H++HSO3-=SO2��+H2O��
���� ������Ҫ�������Ԫ�ص��ƶϣ����Ԫ�����ڱ���������Ԫ���Լ�����������ʣ��������ݱȽϼ�ֻҪ�Ƶ�����Ԫ�ؼ��ɣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���Al��OH��3 ��NaHCO3 �ۣ�NH4��2S ��NaHSO4��
| A�� | ֻ�Т� | B�� | �ٺ͢� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
| A�� | n+3 | B�� | n-4 | C�� | n-6 | D�� | n-8 |
| ѡ�� | ʵ����� | ʵ������ | ���ۻ���� |
| A | ��ij��Һ�м��������ữ��BaCl2��Һ | ���ְ�ɫ���� | ����Һ��һ������SO42- |
| B | ��Ũ�Ⱦ�Ϊ0.1mol•L-1NaCl��NaI�����Һ�еμ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp��AgCl����Ksp��AgI�� |
| C | �����ݵ�������Һ�зֱ�μӱ���NaCl��Һ��CuSO4��Һ | ���г������� | �����ʾ��������� |
| D | ��ij��Һ�еμ�NaOHϡ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ����Һ����NH4+ |
| A�� | A | B�� | B | C�� | C | D�� | D |

| A�� | ���Ӱ뾶��M-��Z2-��Y- | |
| B�� | ZԪ���γɵ������ﶼ�ǹ��ۻ����� | |
| C�� | ��̬�⻯���ȶ��ԣ�Y��Z��M | |
| D�� | ����Ԫ���У�M������������Ӧ��ˮ����������ǿ |
| X | ||
| Y | Z | R |
| W |
| A�� | Z����������X���ʲ����ܷ����û���Ӧ | |
| B�� | ����Ԫ�ص�ԭ������������һ��������2 | |
| C�� | X��Zԭ�������������18 | |
| D�� | Z��������Ԫ�� |
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��SO2ͨ����ɫʯ����Һ�� | ��ɫ��ȥ | SO2����Ư���� |
| B | ��Ũ����ε�pH��ֽ�� | ��ֽ��� | Ũ����������� |
| C | ������NO2���ܱղ������������ˮ�� | ����ɫ���� | ��Ӧ2NO2?N2O4 �ġ�H��0 |
| D | �����ھƾ��ƻ����ϼ��� | �����ۻ��������� | ���������������۵������ |
| A�� | A | B�� | B | C�� | C | D�� | D |

�����й�˵����ȷ���ǣ�������
| A�� | ������м��ٵ�3 g����һ���ǻ���� | |
| B�� | ��������������ٵĹ�������һ����Fe2O3 | |
| C�� | ���������������Եó���ɫ��Һ��n��Cu2+��=0.02 mol | |
| D�� | ���ݲ��������ж�X������������������Ϊ50% |