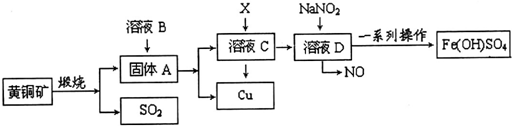
��Ŀ����
5��Ϊ�о��������ƣ�NaClO2������SO2�������ʣ�ij����С�������ͼ��ʾʵ�飨���Ⱥͼг�װ����ȥ����
��1����װ���з�����Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2�����۲쵽��װ������Һ��ɫ��dzʱ��������ֹͣʵ�飮
��3��̽������������SO2�ķ�Ӧ��
�ټ���SO2����ȫ��Ӧ��������װ�������ɵ�SO42-��C1-��Ӧѡ�õ��Լ���ade��������������ClO2-�����ż��飩��
a��ϡ���� b��ϡ���� c��BaCl2��Һd��Ba��NO3��2��Һ e��AgNO3��Һ
�������װ���з�Ӧ�����ӷ���ʽ����SO2+��ClO${\;}_{2}^{-}$+��H2O�T��SO2-+��Cl-+����
��4���ⶨ�������Ƶ������ʣ�ʵ�鲽�����£�
������װ������Һȡ�����������KI���壬�ٵ�������ϡ���ᣬ��ַ�Ӧ��ClO2-+4I-+4H+=2H2O+2I2+C1-����
�������袡������Һϡ����100.00mL��ȡ25.00mL����ƿ�У�����1��2�ε�����Һ����0.500mol•L-1Na2S2O3��Һ�ζ���I2+2S2O32-=2I-+S4O62-�����ظ�����3�εζ�����Na2S2O3��Һ�����ƽ��ֵΪ20.00mL��
�ٲ��袢�н���Һϡ����100.00mL����Ҫ�õ��IJ����������ձ�����������100mL����ƿ����ͷ�ιܣ�
�ڸ�ʵ��NaClO2��������Ϊ90.0%��
��5��ʵ���������С��ͬѧ��������Ϊ������SO2���ܽ��������������NaClO2�������ʣ�����SO2���ܽ����Ĵ�ʩ���ʵ������¶ȣ�����������Ҳ�ɣ�����һ�֣���
���� ��1��Ũ������Cu����ʱ��Ӧ����������ͭ���������ˮ��
��2����װ����β��������������װ�ã�����������л�ԭ�ԣ��ܹ������Ը���������������������Һ��ɫ��dz��
��3����NaClO2��Һ�Լ��ԣ�����SO42-��C1-��Ӧ���������ữ���ټ��뱵���Ӻ������ӣ�ע�ⲻ�ܴ��������ӣ�
�ھݵ�ʧ�����غ��ԭ���غ���ƽ��ѧ����ʽ��
��4���ٽ���Һϡ����100.00mL����Ҫ�IJ����������ձ�����������100mL������ƿ�ͽ�ͷ�ιܣ�
��50mL2mol/L��NaClO2��Һ�к���n��NaClO2��=0.05L��2mol/L=0.1mol���ݵζ�����ͷ���ʽ����ʣ��NaClO2�����ʵ���������NaClO2�������ʣ�
��5��������ܽ�����¶ȵ����߶���С�����¿����������������ܽ�ȣ�
��� �⣺��1��Ũ������Cu����ʱ��Ӧ����������ͭ���������ˮ����ѧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2�����������Һ��ɫ��dz��˵����װ���в������ն���������ֹͣ���飬�ʴ�Ϊ����Һ��ɫ��dz��
��3���ټ���SO42-��C1-��Ӧ���������ữ���ټ��뱵���Ӻ������ӣ������������ữ���������Ȼ����������������Ϊ�ܹ����������ӣ�ѡ�����ᱵ��������������������������ӣ��ʴ�Ϊ��ade��
����ClO2-��ClΪ+3�ۣ���Ӧ��Ϊ-1�ۣ�1molClO2-��4mol���ӣ�SO2��SΪ+4�ۣ���Ӧ��Ϊ+6�ۣ�1molSO2ʧ2mol���ӣ�����ClO2-��SO2�����ʵ���֮��Ϊ1��2���پ�ԭ���غ���ƽ���ӷ���ʽΪ2SO2+ClO2-+2H2O�T2SO2-+Cl-+4H+���ʴ�Ϊ��2��1��2��2��1��4H+��
��4���ٽ���Һϡ����100.00mL����Ҫ�IJ����������ձ�����������100mL������ƿ�ͽ�ͷ�ιܣ��ʴ�Ϊ��100 mL����ƿ����ͷ�ιܣ�
�ھݵζ��������ӦClO2-+4I-+4H+=2H2O+2I2+C1-����I2�����ʵ���Ϊ0.500mol/L��0.020L��2��4=0.02mol���о���Ӧ����ʽClO2-+4I-+4H+=2H2O+2I2+C1-��֪��û�вμ�2SO2+ClO${\;}_{2}^{-}$+2H2O�T2SO2-+Cl-+4H+��Ӧ��ClO${\;}_{2}^{-}$Ϊ0.01mol����Ӧ��0.09mol��50mL2mol/L��NaClO2��Һ�к���n��NaClO2��=0.05L��2mol/L=0.1mol��NaClO2��������Ϊ$\frac{0.09mol}{0.1mol}$��100%=90.0%���ʴ�Ϊ��90.0%��
��5��������ܽ�����¶ȵ����߶���С�����¿����������������ܽ�ȣ��ʴ�Ϊ���ʵ������¶ȣ�����������Ҳ�ɣ���
���� ���⿼���˻�ѧ����ʽ��д�����Ӽ��顢������ԭ��Ӧ����ʽ��ƽ���ζ����顢������ԭ��Ӧ�ļ��㡢������ܽ�ȣ���Ŀ�ѶȽϴ�

| A�� | XԪ�صĺ��������Ϊ288 | |
| B�� | ����${\;}_{115}^{288}$X����������������֮��Ϊ173 | |
| C�� | 113��Ԫ�����ڷǽ���Ԫ�� | |
| D�� | 115��Ԫ�ص���������ϼ���+5 |
| A�� | �������Ƶĵ���ʽ�� | |
| B�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{80}Br$ | |
| C�� | �����ӵĽṹʾ��ͼ�� | |
| D�� | NH3 �ĵ���ʽ�� |
| A�� | $\frac{a+b}{4}$mol | B�� | $\frac{a+c}{4}$mol | C�� | $\frac{3a+c}{4}$mol | D�� | cmol��$\frac{b}{3}$mol |
| A�� | �������м���ŨH2SO4����ַ�������˵��Ũ���������ˮ�� | |
| B�� | Ũ���ᱣ������ɫ��ϸ��ƿ�У������ڵ��������� | |
| C�� | ��ζ����ȶ������ȷֽⶼ���ɰ��� | |
| D�� | ��Fe��NO3��2��Һ�еμ�ϡ���ᣬ�����Եı仯 |


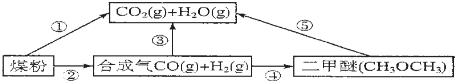
 ��
��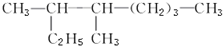 3��4-����-���飮
3��4-����-���飮