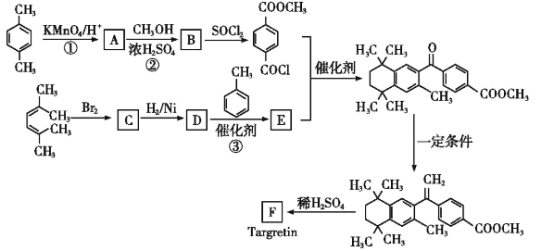
��Ŀ����
����Ŀ�������仯���������������������Ź㷺��Ӧ�á�
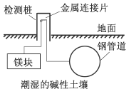
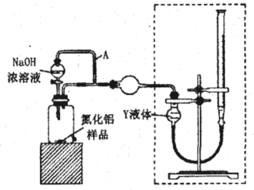
(1)ij�о���ѧϰС�����������ͼ��ʾװ��̽�������ĸ�ʴ��������ձ���Һ���Ϊ����ʳ��ˮ��
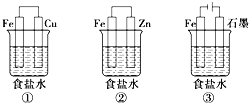
������ͬ�����£�����װ�������缫��ʴ������__(��װ�����)����װ����������ӦʽΪ__��
��Ϊ��ֹ����Fe����ʴ�����Բ�������___(��װ�����)װ��ԭ�����з�����
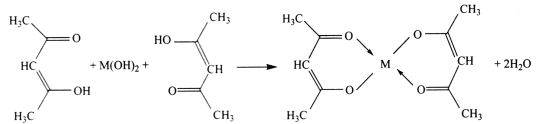
(2)ͨ����������﮵�������С�������ᡢ��������ͻ�����ɸ߱��ʳ�ŵ硢��ɫ�������ڶ��ŵ㡣������﮵�������������Ϊ�������ϵ�һ������Ӷ��ε�أ��ŵ�ʱ��������ӦʽΪM1-xFexPO4+e-+Li+=LiM1-xFexPO4����ԭ����ͼ��ʾ��
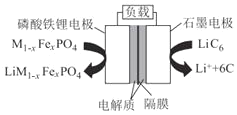
���ŵ�ʱ��������___�缫����������___�缫��������ӦʽΪ____��
���õ�ع���ʱLi+����___�缫�����ʱʯī�缫�ӵ�Դ��____����
���õ�ص��ܷ�Ӧ����ʽΪ____��
���𰸡��� O2+2H2O+4e�� = 4OH�� ���� ������� ʯī LiC6��e�� = Li++6C ������� �� M1-xFexPO4+LiC6X![]() LiM1-xFexPO4+6C
LiM1-xFexPO4+6C
��������
(1)��������������ͭΪ����������пΪ��������Ϊ������������������������������������Ϊ������ʯīΪ����������ӵ�Դ��������������
(2)���ŵ�ʱ��ʯī�缫LiC6ʧȥ���ӱ�Ϊ����ӣ�Ϊ���������Ϊ��������ԭ������������������ƶ������������ƶ��������������缫��Ӧʽ��ӵõ��ܷ�Ӧ����ʽ��
(1)������ͬ�����£���������������ͭ��������������ʴ����ʴ�Ͽ죬����пΪ��������Ϊ���������ܵ�һ��������������������������������������Ϊ������ʯīΪ���������ܵ�����������ӵ�Դ���������������������װ�������缫��ʴ������������װ����������ʴ�����������ӦʽΪO2+2H2O+4e�� = 4OH�����ʴ�Ϊ������O2+2H2O+4e�� = 4OH����
������ǰ������õ�Ϊ��ֹ����Fe����ʴ�����Բ�����������װ��ԭ�����з������ʴ�Ϊ���ڢۡ�
(2)���ŵ�ʱ��ʯī�缫LiC6ʧȥ���ӱ�Ϊ����ӣ�����������˵������������(����)�缫����������ʯī(����)�缫��������ӦʽΪLiC6��e�� = Li++6C���ʴ�Ϊ��������ﮣ�ʯī��LiC6��e�� = Li++6C��
��ԭ������������������ƶ������������ƶ�����˸õ�ع���ʱLi+����������������﮵缫���ŵ�ʱ��ʯī�缫Ϊ����������ʱʯī�缫�ӵ�Դ�ĸ������ʴ�Ϊ��������ﮣ�����
���õ�صĸ�����Ӧ��LiC6��e�� = Li++6C��������Ӧ��M1-xFexPO4+e��+Li+=LiM1-xFexPO4������ܷ�Ӧ����ʽΪM1-xFexPO4+LiC6X![]() LiM1-xFexPO4+6C���ʴ�Ϊ��M1-xFexPO4+LiC6X
LiM1-xFexPO4+6C���ʴ�Ϊ��M1-xFexPO4+LiC6X![]() LiM1-xFexPO4+6C��
LiM1-xFexPO4+6C��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ����л��ϳ��о��й㷺Ӧ�á�
(1)�ü״���ȡ�װ��ķ�ӦΪ��CH3OH(g)+NH3(g)CH3NH2(g)+H2O(g)��H
��֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C�DO | H�DO | N�DH | C�DN |
���ܣ�kJ��mol-1 | 351 | 463 | 393 | 293 |
��÷�Ӧ�ġ�H=______kJ��mol-1
(2)һ�������£���2mol CO��6mol H2ͨ��2L�ܱ������з������·�Ӧ��
����Ӧ��CO(g)+2H2(g)CH3OH(g)��H<0 ��
����Ӧ��2CH3OH(g)CH3OCH3(g)+H2O(g)��H<0 ��
��Ӧ��t minʱ���ﵽƽ��״̬��ƽ��ʱCH3OH�����������(CH3OH)���¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
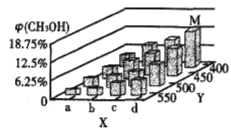
��ͼ��a___b(��������������С����)��ͼ��Y���ʾ�¶ȣ���������_________��
������ӦII��ƽ�ⳣ��Kֵ��С��������˵������ȷ����___________(�����)��
A��ƽ���������Ӧ�����ƶ� B��ƽ���ƶ���ԭ�����������¶�
C���ﵽ��ƽ�����(CH3OH)��С D����������(CH3OCH3)����
��ƽ��ʱ��M��CH3OH���������Ϊ12.5����c(CH3OCH3)=0.1mol��L-1�����ʱCO��ת����Ϊ_____����H2��ʾI�ķ�Ӧ����Ϊ_____mol��L-1��min-1��
(3)��NaOH��Һ��CO2̼�������ڽ���̼�ŷŵ�ͬʱҲ�������Ҫ�Ļ�����ƷNa2CO3�������£���ij�β���õ�pH=11����Һ������Һ��c(![]() )��c(
)��c(![]() )=___________[��֪H2CO3�ĵ���ƽ�ⳣ��Ϊ��K1=4.4��107��K2=5��1011]����Һ��c(Na+)_______ c(
)=___________[��֪H2CO3�ĵ���ƽ�ⳣ��Ϊ��K1=4.4��107��K2=5��1011]����Һ��c(Na+)_______ c(![]() )+2c(
)+2c(![]() )(����>����<������=��)��
)(����>����<������=��)��
����Ŀ��ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
��1���÷�Ӧ�Ļ�ѧ����ʽΪ ________________________________
��2���ӿ�ʼ��2min��Z��ƽ����Ӧ����Ϊ ________________
��3��ij̽����ѧϰС������ͬ������п����ͬŨ�ȵ�������ϡ���ᷴӦ�õ�ʵ���������±���ʾ��
ʵ���� | п��״̬ | ��Ӧ�¶�/�� | �ռ�100mL���� ����ʱ��/s |
�� | ��Ƭ | 15 | 200 |
�� | ��Ƭ | 25 | 90 |
�� | ��ĩ | 25 | 10 |
�ٸ�ʵ���Ŀ����̽�� ____________ �� _______ ��п��ϡ���ᷴӦ���ʵ�Ӱ�죻
��ʵ���͢���� _________________ ����ѧ��Ӧ����Խ��