��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣����ݱ��и�����10��Ԫ�أ���Ҫ������
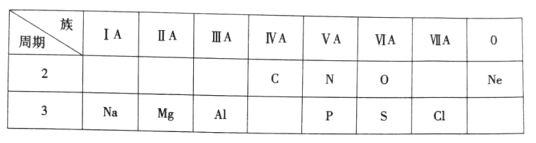
(1)þԪ��λ��Ԫ�����ڱ��е�___����_____�壻
(2)��������������������������_____��
(3)O��S��ȣ�ԭ�Ӱ뾶��С����____��
(4)���ʯ�����Ԫ����________��
(5)���ʵĻ�ѧ��������õ�Ԫ����______��
(6)Na��MgԪ����Ƚ����Խ�ǿ����_________
(7)NH3��PH3��ȣ����ȶ��Խ�������______
(8)H2SO4��H3PO4������Խ�ǿ����______
(9)Mg(OH)2��Al(OH)3�����������������������________
(10)���ʳʻ���ɫ���������Ԫ����_____���䵥�ʵ�ˮ��Һ��__(�������Ի���������)��
���𰸡�3 ��A N2 O C Ne Na PH3 H2SO4 Al(OH)3 Cl ����
��������
(1)þԪ�ص�ԭ������Ϊ12����������ӣ�����������Ϊ2��λ��Ԫ�����ڱ��е�3������A�壻
(2)����������������ռ78%���ǵ���������Ϊ21%��ϡ������0.94%����������0.03%��������̼0.03%���������������������N2��
(3)O��Sλ��ͬһ���壬ͬ����Ԫ�ش��ϵ��°뾶������O��S��ԭ�Ӱ뾶��С����O��
(4)���ʯ��̼Ԫ���γɵĵ��ʣ����Ԫ����C��
(5)ϡ���������������ã����ʵĻ�ѧ��������õ�Ԫ����Ne��
(6)Na��Mgλ��ͬһ���ڣ�ͬ����Ԫ�ش����ҽ���������ǿ��Na��MgԪ����Ƚ����Խ�ǿ����Na��
(7)N��Pλ��ͬһ���壬ͬ����Ԫ�ش��ϵ��·ǽ�������������̬�⻯����ȶ��Լ�����NH3��PH3��ȣ����ȶ��Խ�������PH3 ��
(8) P��Sλ��ͬһ���ڣ�ͬ����Ԫ�طǽ���������ǿ��H2SO4��H3PO4������Խ�ǿ����H2SO4��
(9)Mg(OH)2��Al(OH)3�������������������������Al(OH)3�������Ժ�ǿ�ᷴӦ��Ҳ���Ժ�ǿ�Ӧ��
(10)���ʳʻ���ɫ�����������������Ԫ����Cl��������ˮ��Һ����ˮ����������ʹ���������ʣ��䵥�ʵ�ˮ��Һ�����ԡ�

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�����Ŀ����3�������Ϊ2.0L�ĺ����ܱ������У���ӦCO2(g)+C(s)![]() 2CO(g) H��0�ֱ���һ���¶��´ﵽ��ѧƽ��״̬������˵����ȷ���ǣ� ��
2CO(g) H��0�ֱ���һ���¶��´ﵽ��ѧƽ��״̬������˵����ȷ���ǣ� ��
���� | �¶�/K | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
n(CO2) | n(C) | n(CO) | n(CO) | ||
�� | 977 | 0.28 | 0.56 | 0 | 0.4 |
�� | 977 | 0.56 | 0.56 | 0 | x |
�� | 1250 | 0 | 0 | 0.56 | y |
A.�ﵽƽ��ʱ����������������C(s)������ƽ�������ƶ�
B.x=0.8��y��0.4
C.�ﵽƽ��ʱ���������е�CO��ת����С��![]()
D.����ʼʱ���������г���0.1molCO2��0.2molCO��������C(s)����Ӧ��������Ӧ�������
����Ŀ����֪25��ʱ�����ֳ��������Ka���±���ʾ��
����� | H2C2O4 | CH3COOH | HCN | H2CO3 |
���볣����molL-1�� | K1=5.6��10-2 K2=5.4��10-3 | K=1.7��10-5 | K=6.2��10-10 | K1=4.2��10-7 K2=5.6��10-11 |
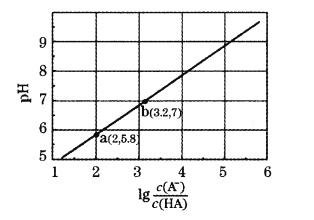
��1�����ᣨH2C2O4����һ��___������һԪ��������Ԫ��������Ԫ�������ᡣ25��ʱ��0.1molL-1��Na2C2O4��CH3COONa��NaCN��Na2CO3��Һ��pH�ɴ�С��˳����__��
��2��KHC2O4��Һ�����ԣ���10mL0.01molL-1��H2C2O4��Һ�μ�0.01molL-1KOH��ҺV(mL)���ش��������⣺
�ٵ�V<10mLʱ����Ӧ�����ӷ���ʽΪ___��
�ڵ�V=10mLʱ����Һ��![]() ��
��![]() ��H2C2O4��H+��Ũ���ɴ�С��˳��Ϊ__��
��H2C2O4��H+��Ũ���ɴ�С��˳��Ϊ__��
�۵�V=amLʱ����Һ������Ũ�������¹�ϵ��c(K+)=2c(![]() )+c(
)+c(![]() )����V=bmLʱ����Һ������Ũ�������¹�ϵ��c(K+)=c(
)����V=bmLʱ����Һ������Ũ�������¹�ϵ��c(K+)=c(![]() )+c(
)+c(![]() )+c(H2C2O4)����a__b������<����=������>����.
)+c(H2C2O4)����a__b������<����=������>����.