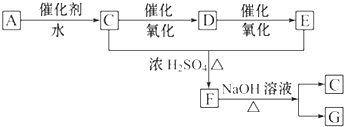
��Ŀ����
7��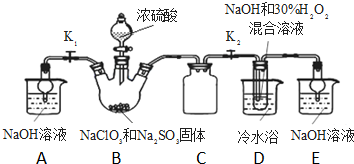 �������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬�ش��������⣺
�������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬�ش��������⣺ʵ�����ȡNaClO2���尴��ͼװ�ý�����ȡ��
��֪��NaClO2������Һ�ڵ���38��ʱ����NaClO2•3H2O������38��ʱ����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��
��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ�IJ�������������������ͷ�ιܡ��ձ��⣬����Ҫ��Ͳ�����������ƣ���װ��C�������Ƿ�ֹDƿ��Һ������Bƿ�У�
��2��װ��D�з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2����Ӧ�����Һ�������ӳ���ClO2-��ClO3-��Cl-��ClO-��OH-����ܺ��е�һ����������SO42-��������ӵķ�����ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-
��3���벹���װ��D��Ӧ�����Һ�л��NaClO2����IJ������裮
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ�����38�桫60����ˮϴ�ӣ��ܵ���60�����õ���Ʒ��
��4�������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
ʵ�����Ʒ���ʷ����봿�Ȳⶨ
��5������ʵ���Ƶõ�NaClO2�����к�����Na2SO4������Na2SO4����ܵ�ԭ����a��
a��B����SO2������������в��ֽ���Dװ����
b��B��Ũ����ӷ�����D����NaOH�к�
c��B�е������ƽ��뵽Dװ����
��6���ⶨ��Ʒ��NaClO2�Ĵ��ȣ��ⶨʱ��������ʵ�飺
ȷ��һ����������Ʒ��������������ˮ������KI���壬�����������·������·�Ӧ��ClO2-+4I-+4H+=2H2O+2I2+Cl-�������û��Һϡ�ͳ�100mL������Һ��ȡ25.00mL������Һ�����������Һ��ָʾ������c mol•L-1 Na2S2O3��Һ�ζ����յ㣬������ı���Һ�����ƽ��ֵΪV mL����֪��I2+2S2O32-=2I-+S4O62-����
��ȷ�ϵζ��յ�������ǵμ����һ��Na2S2O3��Һʱ����Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
������ȡ����Ʒ��NaClO2�����ʵ���Ϊc•V•10-3mol��
���� ��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ�
װ��D�з������巴Ӧ��װ����ѹǿ���ͣ�װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
��2��װ��B���Ʊ��õ�ClO2��װ��D��Ӧ�����Һ���NaClO2���壬װ��D������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��д����ʽ��
B�Ƶõ������к���SO2����װ��D�б������������ᣬ�������������ᱵ�ǰ�ɫ���������������
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����ע���¶ȿ��ƣ�
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl��
��5��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�
��6���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
�ڸ��ݻ�ѧ��Ӧ�ɵù�ϵʽ��NaClO2��2I2��4S2O32-������Ʒ��NaClO2�����ʵ���x�����ݹ�ϵʽ���㣮
��� �⣺��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ����Ի���Ҫ��Ͳ��
װ��D�з������巴Ӧ��װ����ѹǿ���ͣ�װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
�ʴ�Ϊ����Ͳ����ֹDƿ��Һ������Bƿ�У�
��2��װ��B���Ʊ��õ�ClO2��װ��D��Ӧ�����Һ���NaClO2���壬װ��D������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��ʽΪ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
B�Ƶõ������к���SO2����װ��D�б������������ᣬ��Һ�п��ܴ���SO42-�����Ȼ�����Һ����SO42-�����������ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��
�ʴ�Ϊ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��SO42-��ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2•3H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�����ϴ�ӣ�����60����
�ʴ�Ϊ�����ȹ��ˣ���38�桫60����ˮϴ�ӣ�����60����
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl�����������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
�ʴ�Ϊ��NaClO3��NaCl��
��5��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�Ũ�����ѻӷ������������ѻӷ����Σ��������D����a��ȷ��b��c����ѡ��a��
��6���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣬
�ʴ�Ϊ���μ����һ��Na2S2O3��Һʱ����Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
������Ʒ��NaClO2�����ʵ���x����
NaClO2��2I2��4S2O32-��
1mol 4mol
0.25x c mol•L-1��V��10-3L
��x=c•V•10-3mol
�ʴ�Ϊ��c•V•10-3mol��
���� ���⿼�����������Ʊ�ʵ��Ļ����������������Ƶ����ʼ��к͵ζ���֪ʶ������ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢���������������ѶȽϴ�

| A�� | FeO��Fe2O3��Na2O2��Ϊ���������� | B�� | ϡ���������ᡢ�Ȼ�����Һ��Ϊ���� | ||
| C�� | �ռ�����ᡢ���Ȼ�̼��Ϊ����� | D�� | ���ᡢˮ��������ˮ��Ϊ����� |
����֪��Na2S2O3+H2SO4=Na2SO4+S��+SO2+H2O��ij�о�С�����ݸ÷�Ӧ̽����������Է�Ӧ���ʵ�Ӱ�죬���ʵ�����£�
| ʵ�� ��� | ʵ���¶� /�� | Na2S2O3 | H2SO4 | ����ˮ��� /mL | ||
| ���/mL | Ũ��/mol•L-1 | ���/mL | Ũ��/mol•L-1 | |||
| �� | 25 | 10 | 0.1 | 10 | 0.1 | 0 |
| �� | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 25 | 5 | 0.2 | 10 | 0.2 | 5 |
| �� | 50 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 50 | 10 | 0.2 | 5 | 0.2 | 5 |
A��ʵ��ٺ͢�̽��������������ʱNa2S2O3Ũ�ȶ���ط�Ӧ���ʵ�Ӱ��
B��ʵ��ٺ͢���Һ����ǵ�ʱ����ͬ
C��������������ʱ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬Ӧѡ��ʵ��ۺ͢�
D����ͬѧ��ʵ���в��õ��о�������ʵ��ȽϷ�
��ʵ������SO2ͨ��Na2S��Na2CO3�Ļ����Һ�����Ʊ���������ƣ���Ӧԭ��Ϊ��
2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2
��ʵ���Na2S����Ҫ��ϸߣ�����ͼ1��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ����֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ����Ϊ�����ѳ����õĹ�ҵ��Na2S����Բ����ƿ�У�����һ�������ľƾ�������ˮ����ͼ1��ʾװ��������������������ͨ����ȴˮ��ͬʱˮԡ���ȣ�����ƿ�й��岻�ټ���ʱ��ֹͣ���ȣ�����ƿȡ�£��������ȹ��ˣ�����ȴ�ᾧ�����ˣ������ù���ϴ�ӡ�����õ�Na2S•9H2O���壮
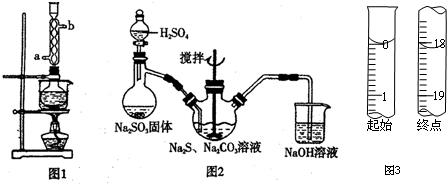
��1�����ᴿ�����С����ȹ��ˡ�������Ŀ���Ƿ�ֹ���ƽᾧ��������ʧ��ȥ�����ʣ�
��2����ͼ2��ʾװ����ȡNa2S2O3������ʢ��Na2SO3����IJ�������������������ƿ��NaOH��Һ������������SO2��β������ֹ��Ⱦ��
��3�����շ���Ʒ��Na2S2O3•5H2O�Ĵ��ȣ�������������ͨ��������ԭ�ζ����ⶨ����ط�Ӧ����ʽΪ2Na2S2O3+I2=2NaI+Na2S4O6��ȷ��ȡW��g��Ʒ����ƿ�У�����������ˮ�ܽ⣬���μӵ�����Һ��ָʾ������0.1000��mol•L?1��ı���Һ���еζ���
��ش�
�ٵ���ζ��յ�ı�־�������һ�ε�ı���Һ����Һ��Ϊ��ɫ����30s�ڲ��ָ�ԭɫ��
�ڵζ���ʼ���յ��Һ��λ����ͼ3�������ĵ�ı���Һ���Ϊ18.10mL����Ʒ�Ĵ���Ϊ$\frac{0.362M}{W}$%����Na2S2O3•5H2O��Է�������ΪM����
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3•5H2O�Ĵ��ȵIJ������ƫ�ͣ��ƫ�ߡ�����ƫ�͡����䡱����
| A�� | ������ʹ����KMnO4��Һ��ɫ��������������ƣ���˱�Ϊ������ | |
| B�� | ���Ľṹ��ʽΪ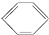 ������˫����������ˮ�����ӳɷ�Ӧ ������˫����������ˮ�����ӳɷ�Ӧ | |
| C�� | ����6��̼ԭ�Ӻ�6����ԭ����ͬһƽ���� | |
| D�� | ��1 mL����1 mLˮ��ֻ�Ϻ��ã������� |
| NaCl | MgCl2 | AlCl3 | SiCl4 | ����B | |
| �۵�/�� | 810 | 710 | 190 | -68 | 2 300 |
| �е�/�� | 1 465 | 1418 | 182.7 | 57 | 2 500 |
| A�� | SiCl4�Ƿ��Ӿ��� | B�� | ����B������ԭ�Ӿ��� | ||
| C�� | AlCl3���������� | D�� | KCl���۵����810�� |