��Ŀ����
15��I��CH4��CO2���������ֵ���ߵĻ�ѧ��Ʒ����֪��CH4��g��+2O2��g��?CO2��g��+2H2O��g����H1=a kJ/mol
CO��g��+H2O��g��?CO2��g��+H2��g����H2=b kJ/mol
2CO��g��+O2��g��?2CO2��g����H3=c kJ/mol
��1����ӦCH4��g��+CO2��g��?CO��g��+2H2��g����H=a+2b-2c kJ/mol���ú�a��b��c�Ĵ���ʽ��ʾ����
��2��һ�������£������ʵ����ģ�1���з�Ӧ���ɵ�����ɺϳɶ����ѣ�CH3OCH3����ͬʱ��������һ�ֿɲ������ѭ������������÷�Ӧ�Ļ�ѧ����ʽΪ3CO+3H2�TCH3OCH3+CO2��
��3����Cu2Al2O4��������һ�������·�����Ӧ��
CO2��g��+CH4��g��?CH3COOH��g�����¶�������Ĵ�Ч�ʺ�������������ʵĹ�ϵ��ͼ���ش��������⣺
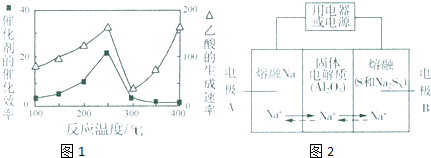
��250��300��ʱ��������������ʽ��͵�ԭ���Ǵ����Ĵ�Ч�ʽ��ͣ���ѧ��Ӧ���ʽ��ͣ�
��300��400��ʱ������������������ߵ�ԭ�����¶����ߣ���ѧ��Ӧ���ʼӿ죮
�������������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ������ͼ��ʾ��
Na2SX$?_{�ŵ�}^{���}$2Na+xS ��3��x��5��
| ���� | Na | S | Al2O3 |
| �۵�/�� | 97.8 | 115 | 2050 |
| �е�/�� | 892 | 444.6 | 2980 |
A��100������ B��100�桫300��
C��300�桫350��D��350�桫2050��
��5�����������أ�����˵����ȷ����AD������ĸ��ţ���
A���ŵ�ʱ���缫AΪ����
B���ŵ�ʱ��Na+���ƶ�����Ϊ��B��A
C�����ʱ���缫AӦ���ӵ�Դ������
D�����ʱ�缫B�ĵ缫��ӦʽΪSX2--2e-=xS
��6��25��ʱ��������������Ϊ��Դ���500mL 0.2mol/L NaCl��Һ������Һ��pH��Ϊl3ʱ����·��ͨ���ĵ��ӵ����ʵ���Ϊ0.05mol�������ķ�Ӧ���������Ϊ2.3 g����������ǰ�����ķ�Ӧ���������ȣ�
���� ��1��������֪�Ȼ�ѧ����ʽ�ʹ����Ȼ�ѧ����ʽ���ݸ�˹���ɽ��
��2���ݷ�Ӧ����������֪���������ѭ������������Ϊ������̼����ԭ���غ���д��ѧ����ʽ��
��3�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ�
���¶����ߣ���ѧ��Ӧ���ʼӿ죻
��4��ԭ��ع���ʱ�����Ƶ��¶�ӦΪ����Na��SΪ����״̬���ݴ˷�����
��5������ͼƬ֪���ŵ�ʱ��Naʧ���ӷ���������Ӧ������A��������B��������������ӦʽΪ2Na-2e-�T2Na+��������ӦʽΪxS+2e-�TSx2-�����ʱAΪ������BΪ�����������������缫��Ӧʽ�븺����������Ӧʽ�����෴���ŵ�ʱ����������������������ƶ������������ƶ���
��6�����NaCl��Һ�ķ�ӦʽΪ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2����ÿ����2molNaOHת�Ƶ���2mol������Һ��pH��Ϊl3ʱ��c��OH-��=0.1mol/L������NaOH���ʵ���Ϊ0.05mol��ת�Ƶ���0.05mol����ת�Ƶ��������������仯��
��� �⣺��1����֪��CH4��g��+2O2��g��?CO2��g��+2H2O��g����H1=a kJ/mol
��CO��g��+H2O��g��?CO2��g��+H2��g����H2=b kJ/mol
��2CO��g��+O2��g��?2CO2��g����H3=c kJ/mol
�ݸ�˹���ɣ���+2����-2���۵ã�CH4��g��+CO2��g��?CO��g��+2H2��g����H=��a+2b-2c��kJ/mol��
�ʴ�Ϊ��a+2b-2c��
��2���ݷ�Ӧ����������֪���������ѭ������������Ϊ������̼����ԭ���غ���д��ѧ����ʽΪ��3CO+3H2�TCH3OCH3+CO2��
�ʴ�Ϊ��3CO+3H2�TCH3OCH3+CO2��
��3�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ��ʴ�Ϊ�������Ĵ�Ч�ʽ��ͣ���ѧ��Ӧ���ʽ��ͣ���14�֣�
���¶����ߣ���ѧ��Ӧ���ʼӿ죬�ʴ�Ϊ���¶����ߣ���ѧ��Ӧ���ʼӿ죻
��4��ԭ��ع���ʱ�����Ƶ��¶�ӦΪ����Na��SΪ����״̬�����¶�Ӧ����115�������444.6�棬ֻ��c���ϣ��ʴ�Ϊ��C��
��5��A��ͨ�����Ϸ���֪�����ʱA��������Ӧ�����ӵ�Դ��������A��ȷ��
B���ŵ�ʱ��B��������A�Ǹ�����Na+��A��B�ƶ�����B����
C�����ʱ���缫A������ԭ��Ӧ��Ӧ���ӵ�Դ�ĸ�������C����
D�����ʱ��B���������缫��ӦʽΪSx2--2e-�TxS����D��ȷ��
�ʴ�Ϊ��AD��
��6�����NaCl��Һ�ķ�ӦʽΪ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2����ÿ����2molNaOHת�Ƶ���2mol������Һ��pH��Ϊl3ʱ��c��OH-��=0.1mol/L������NaOH���ʵ���Ϊ0.05mol��ת�Ƶ���0.05mol��ת��0.05mol���ӣ�����Na��������1.15g�����������仯1.15g�������ķ�Ӧ���������Ϊ2.3g���ʴ�Ϊ��0.05��2.3��
���� ���⿼���˸�˹���ɵ�Ӧ�á�������ԭ��Ӧ����ʽ��д��Ӱ�췴Ӧ���ʵ����ء�ԭ��غ͵��ط���������Ŀ�ѶȽϴ�

| A�� | Ϊ���ũ����IJ�����������Ӧ����ʹ�û��ʺ�ũҩ | |
| B�� | �����Ƿ������绹�ǻ������磬���ǽ���ѧ��ת��Ϊ���� | |
| C�� | PM2.5���е�Ǧ������������ȶ������к��Ľ���Ԫ�� | |
| D�� | ú��ʯ�͡���Ȼ����һ����Դ |
| X | |||
| Z | Y | W |
| A�� | X��Y��W�������ﶼ�������������� | |
| B�� | ��ҵ�ϲ��õ��Z���Ȼ����Ʊ�Z���� | |
| C�� | W�ĵ���������XW2 | |
| D�� | 1molX��Y���������������ж�����2mol˫�� |
| A�� | ������������ | B�� | ����������ԭ | C�� | ����������ԭ | D�� | ������������ |
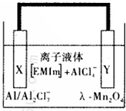 ����һ�ָ��������壬�ǿ�����ص�����缫���ϣ���ͼ��ʾ�����������ε�أ������л������ӣ�EMlm���� AlCl4-��ɵ�����Һ��Ϊ���Һ���йط�ӦΪAl+Mn2O4$?_{���}^{�ŵ�}$AlMn2O4 ����˵����ȷ���ǣ�������
����һ�ָ��������壬�ǿ�����ص�����缫���ϣ���ͼ��ʾ�����������ε�أ������л������ӣ�EMlm���� AlCl4-��ɵ�����Һ��Ϊ���Һ���йط�ӦΪAl+Mn2O4$?_{���}^{�ŵ�}$AlMn2O4 ����˵����ȷ���ǣ�������| A�� | ��طŵ�ʱ��Ac-4-��Y����ɢ | |
| B�� | ��طŵ�ʱ��X������ | |
| C�� | ��س��ʱX���ķ�ӦΪ��4Al2Cl7-+3e-=Al+7AlCl4- | |
| D�� | ����Ϊ�������ϵı���������� |
| A�� | 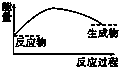 | B�� | 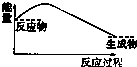 | C�� | 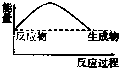 | D�� | 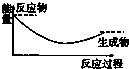 |
| �� ���� | IA | IIA | IIIA | ��A | ��A | ��A | VIIA | 0 |
| 1 | �� | |||||||
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� |
��2���ɢ١��ܡ��ޡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��
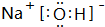 ��
��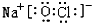 ��
����3���ڡ��ۡ������ۺ������������ǿ������˳����HClO4��HNO3��H2CO3�����ѧʽ��
��4���������γ��������������Ԫ����Al����Ԫ�ط��ţ����ֱ�д����Ԫ�ص�����������ޡ�������������ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��Al��OH��3+3H+=Al3++3H2O��
��5�������һ��ʵ�鷽�����ȽϢܡ���������Ե�ǿ��������������ͽ��ۣ���H2S��Һ��ͨ��O2������Һ���ֻ��ǣ�˵��O2�������Ա�Sǿ��
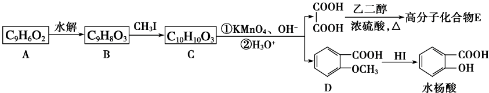
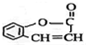 ��
�� ��
��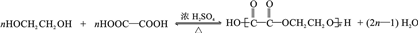 ��
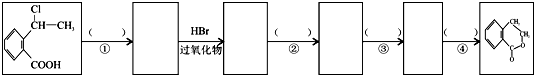
��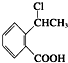 Ϊԭ����
Ϊԭ���� �ĺϳ�·������ͼ�����Լ����ã����ڷ����������Ӧ�����ʣ���������ע����Ӧ�������ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ڷ����������Ӧ�����ʣ���������ע����Ӧ�������ϳ�·������ͼʾ�����£�