��Ŀ����
��һ�������������NaI��KCl��Na2CO3��Na2SO4��CaCl2��Cu(NO3)2�е�һ�ֻ�����ɣ�Ϊ�˼������������ʣ���������ʵ�飺
��ȡ������������ˮ���õ���ɫ����Һ��
��������Һ�еμ��Ȼ�����Һ���а�ɫ�������ɣ�
�۹��ˣ��������м���������ϡ���ᣬ���ֳ���û��ȫ���ܽ�������ɫ��ζ���������ɡ�
������Һ�м������������Ƶ���ˮ���ټ����������ͣ������ã��ϲ�Һ����Ϻ�ɫ��
��1�����жϣ����������п϶����� ��һ��û�� �����ܺ���________________��
��2���Կ��ܺ��е����ʣ���ν���ʵ���Խ�һ�����顣 ��
��3��ʵ����з����Ļ�ѧ��Ӧ���� ��Ӧ���Ӧ���ͣ�����Ҫʵ��������ƽ� ��
��1���϶�����Na2CO3��Na2SO4��NaI��һ��û��Cu(NO3)2��CaCl2�����ܺ���KCl
��2������ɫ��Ӧ��������ɫ�ܲ�������ɫ���棬��KCl����֮����û�С�
��3���û���Ӧ��������ԭ��Ӧ ��ȡ
��10�֡���1��2С��ÿ��2�֣���3С��ÿ��1�֣�
�������������ȡ������������ˮ���õ���ɫ����Һ��˵��һ��������ͭ���ӣ���û������ͭ��������Һ�еμ��Ȼ�����Һ���а�ɫ�������ɣ��ð�ɫ����ֻ�������ᱵ������һ�����������ƣ���һ��û���Ȼ��ơ����ˣ��������м���������ϡ���ᣬ���ֳ���û��ȫ���ܽ�������ɫ��ζ���������ɣ���˵����ɫ�����л�����̼�ᱵ��̼�ᱵ��������������CO2���塣����Һ�м������������Ƶ���ˮ���ټ����������ͣ������ã��ϲ�Һ����Ϻ�ɫ����˵����Ӧ���е��ʵ����ɣ����һ�������е⻯�ƣ����Ȼ��ز���ȷ���Ƿ��С�
��1�����жϣ����������п϶�����Na2CO3��Na2SO4��NaI��һ��û��Cu(NO3)2��CaCl2�����ܺ���KCl��
��2��Ҫ��֤�Ƿ����Ȼ��أ������ͨ����ɫ��Ӧ����ɡ�������ɫ��Ӧ��������ɫ�ܲ�������ɫ���棬��KCl����֮����û�С�
��3����������ǿ�����ԣ��ưѵ⻯���������ɵ��ʵ⣬ͬʱ�����Ȼ������ɣ��÷�Ӧ�����û���Ӧ��������ԭ��Ӧ�����ʵ��������л��ܼ��У���˸�ʵ���������ȡ��
���㣺�������ӹ�������Ӽ�����й�ʵ����ơ���ȡ�����Լ���Ӧ���͵��ж�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�ij��Һ�н����±������е�5������(������ˮ�ĵ��뼰���ӵ�ˮ��)���Ҹ����ӵ����ʵ�����Ϊ0.1mol��
| ������ | SO42����NO3����Cl�� |
| ������ | Fe3����Fe2����NH4+��Cu2����Al3�� |
������ԭ��Һ�м���KSCN��Һ������������
������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䣻
������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
�Իش��������⣺
��1������ԭ��Һ���ȼ����������ᣬ�ٵ���KSCN��Һ��ʵ��������________________��ʵ��������������Ӧ�����ӷ���ʽ��_________________________��
��2��ԭ��Һ��������������(д���ӷ���)___________________.
��3������ԭ��Һ�м�������NaOH��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù����������________________g��
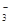 ��SO
��SO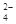 ��NH
��NH ��CO
��CO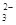 ����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺