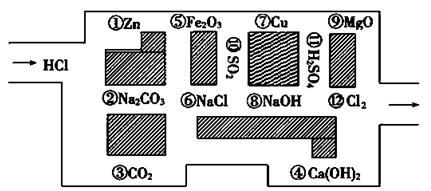
��Ŀ����
��Ҫ��д�����з���ʽ��
��1��������Ͷ��FeCl3��Һ�з�Ӧ�������ӷ���ʽ ��
��2����������Ͷ������ͭ��Һ�������ӷ���ʽ ��
��3��̼���Ⱶ�������������Ʒ�Ӧ�����ӻ�ѧ����ʽ ��
��4��������̼�����Ʒ�Ӧ�����ӷ���ʽ_____________________________________________��
��5��������������������Һ��Ӧ�����ӷ���ʽ_______________________________________��
��6����NaHSO4��Һ��������������Һ�����ԣ�������Ӧ�����Һ�е����������������ӷ���ʽ
��
��1��6Na+6H2O+2Fe3+��6Na++2Fe(OH)3+3H2�� ��2��2Na2O2+2H2O+2Cu2+��O2��+2Cu(OH)2+4Na+
��3��Ba2++2HCO3-+2OH-��BaCO3��+CO32-+2H2O ��4��CH3COOH+HCO3-��CH3COO-+H2O+CO2��
��5��Na2O+2H+��2Na++H2O ��6��Ba2++2H++SO42-+2OH-��BaSO4��+2H2O Ba2++SO42-��BaSO4��
���������������1�����ǻ��õĽ���������ˮ��Ӧ�����������ƺ����������ɵ����������ٺ���Һ�е����ʷ������ֽⷴӦ������������Ͷ��FeCl3��Һ�з�Ӧ�������ӷ���ʽΪ6Na��6H2O��2Fe3+��6Na+��2Fe(OH)3��3H2����
��2����������Ͷ������ͭ��Һ�й�������������ˮ��Ӧ�����������ƺ����������ɵ��������ƺ�����ͭ��Ӧ����������ͭ��ɫ�����������ƣ���������ӷ���ʽΪ2Na2O2��2H2O��2Cu2+��O2����2Cu(OH)2��4Na+��
��3��̼���Ⱶ�������������Ʒ�Ӧ����̼�ᱵ��ɫ������̼���ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2+��2HCO3-��2OH-��BaCO3����CO32-��2H2O��
��4�����������ᣬӦ���û�ѧʽ��ʾ���������̼�����Ʒ�Ӧ�����ӷ���ʽΪCH3COOH+HCO3-��CH3COO-+H2O+CO2����
��5��������������������Һ��Ӧ���������ƺ�ˮ����Ӧ�����ӷ���ʽΪNa2O+2H+��2Na++H2O��
��6����NaHSO4��Һ��������������Һ�����ԣ���ʱ�����������ᱵ�������ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2++2H++SO42-+2OH-��BaSO4��+2H2O��������Ӧ�����Һ�е��������������������Ƽ���������������Ӧ�������ᱵ��ɫ���������Է�Ӧ�����ӷ���ʽΪBa2++SO42-��BaSO4����
���㣺�������ӷ���ʽ����д

ij�����Һ�п��ܺ��е��������±���ʾ:
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32����AlO2�� |
Ϊ̽����ɷ�,����������̽��ʵ�顣
(1)̽��һ:
��ͬѧȡһ�����Ļ����Һ,��������μ�������������Һ,�������������ʵ���(n)���������������Һ�����(V)�Ĺ�ϵ��ͼ��ʾ��
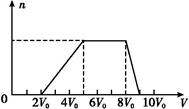
�ٸ���Һ��һ�������ڵ����������� ,
һ�������ڵ�������������������������;���е����������Ӧ���ʵ���Ũ��֮��Ϊ�� ;
����д���������ٹ����з�����Ӧ�����ӷ���ʽ ��
(2)̽����:
��ͬѧ������Һ�к��д�����Cl-��Br-��I-,����1 L�û����Һ��ͨ��һ������Cl2,��Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2�����(��״��)�Ĺ�ϵ���±���ʾ,������ش���������:
| Cl2�����(��״��) | 11.2 L | 22.4 L | 28.0 L |
| n(Cl-) | 2.5 mol | 3.5 mol | 4.0 mol |
| n(Br-) | 3.0 mol | 2.5 mol | 2.0 mol |
| n(I-) | x mol | 0 | 0 |
�ٵ���ʼ��ͨ��Cl2�����Ϊ22.4 Lʱ,��Һ�з�����Ӧ�ܵ����ӷ���ʽΪ�� ;
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ�� ��
��һ��ɫ������Һ��Ҫȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3����SO42����Cl����I����HCO3����ȡ����Һʵ�����£�
| ʵ�鲽�� | ʵ������ |
| ��1��ȡ��������Һ���Ӽ��μ��� | ��Һ���ɫ |
| ��2��ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| ��3��ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| ��4��ȡ��3�����ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| ��5��ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
��1����Һ�п϶����ڵ�������___________________________________________________________����Һ�п϶������ڵ�������__________________________________________________________________��
��2��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ�������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ��������)��_________________________________________________________��
ij�����Һ�п��ܴ������е��������±���ʾ��
| ������ | H����K����Al3����NH4+��Mg2�� |
| ������ | Cl����Br����OH����CO32-��AlO2- |
Ϊ̽����ɷ֣�ijͬѧ��Na2O2���뵽���������Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ�ֱ���ͼ��ʾ��

��1������Һ��һ�����е���������______________����
��Ӧ���ʵ���Ũ��֮��Ϊ________����Һ��һ����
���ڵ���������_____________��
��2����д���������ٵ����ӷ���ʽ_____________________��
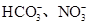 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����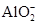 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��