��Ŀ����
����Ŀ�����û�ѧ��Ӧԭ���о�̼�����ĵ��ʼ��仯����ķ�Ӧ�Ի������Ⱦ����ԴΣ��������Ҫ���塣
��1���Ҵ���һ����Ҫ��ȼ�ϣ���ҵ��������ϩ�ƾƾ���C2H4(g)+H2O(l)=C2H5OH(l) ��H����֪��ϩ���Ҵ���ȼ���ȷֱ���1411.0kJ��mol-1��1366.8 kJ��mol-1����H=_____________��
��2����Cu2Al2O4��������һ�������·�����Ӧ��CO2(g)+CH4(g)![]() CH3COOH(g)���¶�������Ĵ�Ч�ʺ�������������ʵĹ�ϵ��ͼ���ش��������⣺
CH3COOH(g)���¶�������Ĵ�Ч�ʺ�������������ʵĹ�ϵ��ͼ���ش��������⣺
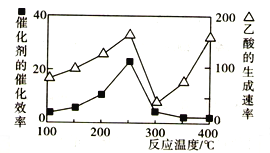
��200~250��ʱ������������������ߵ���Ҫԭ����____________________________��
��300~400��ʱ������������������ߵ���Ҫԭ����____________________________��
��3���״���Ϊһ����Ҫ�Ļ���ԭ�ϡ���һ�������¿����ü״��ʻ�������ȡ����������䷴Ӧԭ���ɱ�ʾΪCH3OH(g)+CO(g)![]() HCOOCH3(g) ��H=-29.1kJ/mol�������Ϊ1L���ܱ������г���3mol CH3OH(g)��3mol CO(g)����������ڵ�ѹǿ(p: kPa) ��ʱ��(t: min) �ı仯��ϵ��ͼ�Т�������ʾ��
HCOOCH3(g) ��H=-29.1kJ/mol�������Ϊ1L���ܱ������г���3mol CH3OH(g)��3mol CO(g)����������ڵ�ѹǿ(p: kPa) ��ʱ��(t: min) �ı仯��ϵ��ͼ�Т�������ʾ��
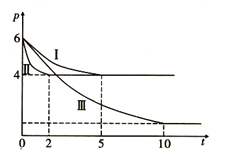
�٢�͢���ȣ����иı�ķ�Ӧ������_______________________��
�ڢ�͢���ȣ����иı�ķ�Ӧ������_________________���жϵ�������________________________��
�۷�Ӧ����5min ʱ�ﵽƽ�⣬�ڴ������´ӷ�Ӧ��ʼ���ﵽƽ��ʱv(CH3OH)= ________________��
�ܷ�Ӧ����2min ʱ�ﵽƽ�⣬ƽ�ⳣ��K(��)= ______________����������¶Ȳ���������£���������Ӧ�ﵽƽ���ʱ�����������м���2mol CH3OH ��1mol HCOOCH3��ƽ��_______�ƶ�(�����������)��ԭ����____________________________________________��
���𰸡� -44.2kJ/mol �����Ĵ�Ч����������ѧ��Ӧ���ʼӿ� �¶����ߣ���ѧ��Ӧ���ʼӿ� ������� ���� ���дﵽƽ�������ʱ����ڢʷ�Ӧ���ʼ������¶ȸ��ͻ���CH3OH(g)+CO(g)![]() HCOOCH3(g)Ϊ���ȷ�Ӧ�������¶�������Ӧ������У�ѹǿ��С 0.4mol(L��min) 2L/mol ���� �¶Ȳ��䣬 ƽ�ⳣ�����䣬Qc=1<K=2���ʷ�Ӧ������Ӧ�����ƶ�
HCOOCH3(g)Ϊ���ȷ�Ӧ�������¶�������Ӧ������У�ѹǿ��С 0.4mol(L��min) 2L/mol ���� �¶Ȳ��䣬 ƽ�ⳣ�����䣬Qc=1<K=2���ʷ�Ӧ������Ӧ�����ƶ�
����������1����ҵ��������ϩ�ƾƾ���C2H4(g)+H2O(l)=C2H5OH(l) ��H����֪��ϩ���Ҵ���ȼ���ȷֱ���1411.0kJ��mol-1��1366.8 kJ��mol-1������ȼ���Ⱦ�����һ��������ÿĦ����ȼ����ȫȼ�������ȶ����������ų�����������������H=��-1411.0kJ��mol-1��-��-1366.8 kJ��mol-1��-44.2kJ/mol��
��2������ͼ����Ϣ��֪��200~250��ʱ������������������ߵ���Ҫԭ���Ǵ����Ĵ�Ч�����ߣ���ѧ��Ӧ���ʼӿ졣
����ͼ��֪��300~400��ʱ�����Ĵ�Ч����С����������������������ߵ���Ҫԭ�����¶�����ʹ��ѧ��Ӧ���ʼӿ졣
��3����һ�������¿����ü״��ʻ�������ȡ����������䷴Ӧԭ���ɱ�ʾΪCH3OH(g)+CO(g)![]() HCOOCH3(g) ��H=-29.1kJ/mol���÷�Ӧ��һ��������������ٵķ��ȷ�Ӧ����������Ӧ�ķ����������ѹǿ��С��
HCOOCH3(g) ��H=-29.1kJ/mol���÷�Ӧ��һ��������������ٵķ��ȷ�Ӧ����������Ӧ�ķ����������ѹǿ��С��
����ͼ����Ϣ��֪����͢���ƽ��״̬�µ�ѹǿ��ͬ�����Ǣ�Ļ�ѧ��Ӧ���ʽϴ����ԣ���͢���ȣ����иı�ķ�Ӧ�����Ǽ��������
�ڢ�͢���ȣ����ƽ����ѹ��С���һ�ѧ��Ӧ���ʽ��������Ԣ��иı�ķ�Ӧ�����ǽ��£��жϵ������������дﵽƽ�������ʱ����ڢʷ�Ӧ���ʼ������¶ȸ���������CH3OH(g)+CO(g)![]() HCOOCH3(g)Ϊ���ȷ�Ӧ�������¶�������Ӧ������У�ѹǿ��С����
HCOOCH3(g)Ϊ���ȷ�Ӧ�������¶�������Ӧ������У�ѹǿ��С����
�۷�Ӧ����5min ʱ�ﵽƽ�⣬�ڴ������´ӷ�Ӧ��ʼ���ﵽƽ��ʱ����CH3OH�ı仯��Ϊx����CO��HCOOCH3�ı仯��Ҳ��x��CH3OH��CO��HCOOCH3��ƽ�����ֱ�Ϊ3mol-x��3mol-x��x����ͼ��֪����Ӧǰ���ѹǿ֮��Ϊ6:4=3:2��������3mol+3mol������3mol-x +3mol-x+x��=3:2����֮�ã�x=2mol����ˣ�v(CH3OH)= ![]() 0.4mol(L��min) ��
0.4mol(L��min) ��
�ܷ�Ӧ����2min ʱ�ﵽƽ�⣬��ƽ�ⳣ���뷴Ӧ����ͬ������ƽ�ⳣ��K(��)= ![]() 2L/mol����������¶Ȳ���������£���������Ӧ�ﵽƽ���ʱ�����������м���2mol CH3OH ��1mol HCOOCH3��ƽ�������ƶ���ԭ�������¶Ȳ��䣬 ƽ�ⳣ�����䣬Qc=1<K=2���ʷ�Ӧ������Ӧ�����ƶ���
2L/mol����������¶Ȳ���������£���������Ӧ�ﵽƽ���ʱ�����������м���2mol CH3OH ��1mol HCOOCH3��ƽ�������ƶ���ԭ�������¶Ȳ��䣬 ƽ�ⳣ�����䣬Qc=1<K=2���ʷ�Ӧ������Ӧ�����ƶ���

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ����ѧ�������о���������CH4��CO2��ת�������á���ش��������⣺
��1������һ���ռ��˶�״̬�ĵ�����ԭ�Ӻ�����ֵĸ����ܶȷֲ�����____________�����������ڻ�̬14Cԭ���У��������___________�������෴�ĵ��ӡ�
��2��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ__________________________��
��3��һ�������£�CH4��CO2������H2O�γ���״�ṹ(����ͼ��ʾ)��ˮ���ᄃ�壬����ز������±���CH4��H2O�γɵ�ˮ�����׳�����ȼ������
���� ���ӡ����� | ����ֱ��/nm | ������H2O�Ľ����E/kJ��mol��1 |
CH4 | 0.436 | 16.40 |
CO2 | 0.512 | 29.91 |

�����й���CH4��CO2��˵����ȷ����________(�����)��
a��CO2�������2��������2������
b��CH4�����к��м��Թ��ۼ����Ǽ��Է���
c����Ϊ̼�������С��̼����������CH4�۵����CO2
d��CH4��CO2������̼ԭ�ӵ��ӻ����ͷֱ���sp3��sp
��Ϊ�������������ȼ�������п�ѧ�������CO2�û�CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0. 586 nm����������ͼ�����ṩ�����ݷ���������������������______________________________________��