��Ŀ����
9������˵����ȷ���ǣ�������| A�� | ��ij�¶��£�0.2 mol/L�Ĵ�����0.09 mol/L�����������������Ϻ�����ԣ������ǻ�Ϻ���Һ����ı仯�������������¶��´���ĵ��볣�� K=9��10-7 mol/L | |
| B�� | ��ij�¶����Ȼ�����Ksp=10-10mol2/L2����100 mL0.2 mol/L���Ȼ�����Һ��0.18 mol/L��������Һ�������Ϻ����ǻ�Ϻ���Һ����ı仯������Һ��c��Ag+��Ϊ10-8 mol/L | |
| C�� | ��ij�¶���1 L�ܱ������з�ӦHCHO��g��ʮH2��g��?CH3OH��g���ﵽƽ����ȩ��ת����Ϊ50%������¶��¸÷�Ӧ��ƽ�ⳣ����2��mol/L��-1 | |
| D�� | �����������Ƶı�����Һ�м�������������Һ������й������� |
���� A���ɵ���غ㣬c��CH3COO-��=2c��Ba2+��=0.09mol/L��c��CH3COOH��=0.1mol/L-0.09mol/L������Ka=$\frac{c��C{H}_{3}CO{O}^{-}����c��{H}^{+}��}{c��C{H}_{3}COOH��}$���㣻
B������Ksp=c��Ag+����c��Cl-�����㣻
C��û�и�����Ӧ��ij�ʼŨ�ȣ������㣻
D������Һ��Qc=c��Ca2+����c2��OH-����Kspʱ���г������ɣ�
��� �⣺A���ɵ���غ㣺c��CH3COO-��+c��OH-��=2c��Ba2+��+c��H+������Һ�����ԣ���Ϊ�����£�����Һ��c��H+��=10-7mol/L����c��CH3COO-��=2c��Ba2+��=0.09mol/L��
c��CH3COOH��=0.1mol/L-0.09mol/L=0.01mol/L��Ka=$\frac{c��C{H}_{3}CO{O}^{-}����c��{H}^{+}��}{c��C{H}_{3}COOH��}$=$\frac{0.09��1{0}^{-7}}{0.01}$=9��10-7 mol/L�������¶Ȳ�֪������Ka��9��10-7����A����
B��100 mL0.2 mol/L���Ȼ�����Һ��0.18 mol/L��������Һ�������Ϻ�Ӧ��ʣ���c��Cl-��=$\frac{0.1��0.2-0.1��0.18}{0.2}$=0.01mol/L��Ksp=c��Ag+����c��Cl-������c��Ag+��=$\frac{1{0}^{-10}}{0.01}$=10-8 mol/L����B��ȷ��
C��û�и�����ȩ�������ij�ʼŨ�ȣ�������ƽ��ʱ��ȩ�������ͼ״���Ũ�ȣ���������ƽ�ⳣ������C����
D������Һ��Qc=c��Ca2+����c2��OH-����Kspʱ���г������ɣ������������Ƶı�����Һ�м�������������Һ�����������������������ӵ�Ũ�Ⱥ�С�����Ϻ�Qc��Ksp������Һ��û�г������ɣ���D����
��ѡB��
���� ���⿼���˵���ƽ�ⳣ���ļ��㡢�ܶȻ������ļ��㡢����غ��Ӧ�õȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�| A�� | 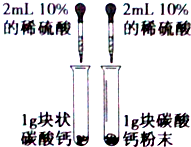 ��ͼװ��̽����Ӧ��Ӵ�����Է�Ӧ����Ӱ�� | |
| B�� |  ��ͼװ����ȡ���ռ�������NO2���壨�����ǵ����� | |
| C�� |  ��ͼװ����ȡ���ռ����������� | |
| D�� |  ��ͼװ����ȡ������������ |
| A�� | C$\stackrel{�ڿ����е�ȼ}{��}$CO$\stackrel{CuO����}{��}$CO2$\stackrel{NaOH��Һ}{��}$Na2CO3 | |
| B�� | Cu$\stackrel{AgNO_{3}��Һ}{��}$Cu��NO3��2��Һ$\stackrel{NaOH��Һ}{��}$Cu��OH��2 | |
| C�� | Fe$\stackrel{��ȼ}{��}$Fe2O3$\stackrel{H_{2}SO_{4}��Һ}{��}$Fe2��SO4��3��Һ | |
| D�� | CaO$\stackrel{H_{2}O}{��}$Ca��OH��2��Һ$\stackrel{Na_{2}CO_{3}}{��}$NaOH��Һ |
| A�� | 0.01mol��L-1H2S��Һ��c��H+����c��HS-����c��S2-����c��H2S����c��OH-�� | |
| B�� | O��lmol��LNaHSO3��Һ��c��Na+��+c��H+����c��HSO3-��+c��SO32-��+c��OH-�� | |
| C�� | �����ʵ���NH4Cl�� NaCl �Ļ����Һ��c��NH4+��+c��NH3��H20��+c��Na+��=2c��Cl-�� | |
| D�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=10-10��Na2CO3��Һ��c��HCO3-��+2c��H2CO3��=��10-2-10-12��mol/L |
| A�� |  ��ͼ��ʾװ�÷��뱽��ˮ | |
| B�� |  ��ͼ��ʾװ�ó�ȥC2H2�к��е�����H2S | |
| C�� |  ��ͼ��ʾװ�÷���NaCl��CaCl2�Ļ����Һ | |
| D�� |  ��ͼ��ʾװ������NH4Cl������Һ�Ʊ�NH4Cl���� |
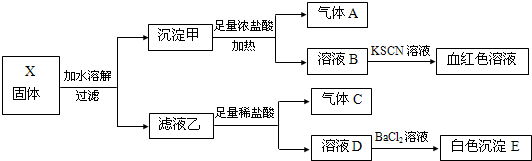
��������ʵ�飬����˵����ȷ���ǣ�������
| A�� | ����X��һ������Fe2O3 | |
| B�� | ����E���ܺ���BaSO3 | |
| C�� | ����A��Cһ����Ϊ������ | |
| D�� | ����X���ܳ��ֺ���K2SO3��K2CO3������Na2SO4����� |
| A�� | ����Se�ڿ�����ȼ�տ�����SeO2 | B�� | Se��ԭ������Ϊ24 | ||
| C�� | ���γ�Na2SeO3��Na2SeO4������ | D�� | H2S��H2Se�ȶ� |
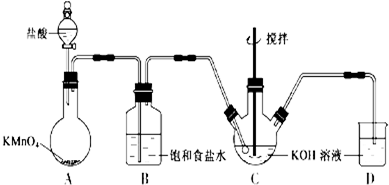

 ��ˮ������С�մ���Һ��Ӧ�Ļ�ѧ����ʽ��
��ˮ������С�մ���Һ��Ӧ�Ļ�ѧ����ʽ��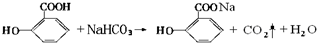 �� һ�������£�C��F��Ӧ�ķ�Ӧ������_������Ӧ��
�� һ�������£�C��F��Ӧ�ķ�Ӧ������_������Ӧ��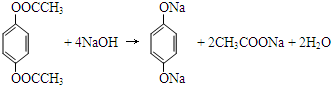 ��
��