��Ŀ����
9����1��NH3��һϵ�з�Ӧ���Եõ�HNO3��NH4NO3����ͼ��ʾ���ڢ��У�NH3��O2�ڴ��������·�Ӧ���仯ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��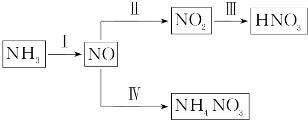
��2�����У�2NO��g��+O2��g��?2NO2��g����������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ��p1��p2�����¶ȱ仯�����ߣ���ͼ����
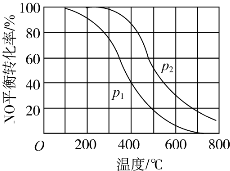
��p1��p2�Ĵ�С��ϵP1��P2��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯�������Ǽ�С
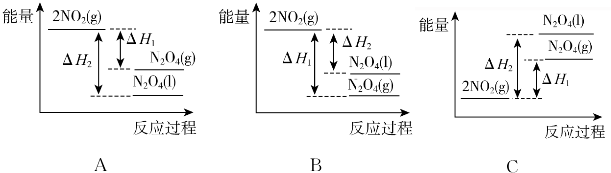
��3�����У������¶ȣ���NO2��g��ת��ΪN2O4��1�����Ʊ�Ũ���ᣮ
����֪��2NO2��g��?N2O4��g����H1 2NO2��g��?N2O4��1����H2
���������仯ʾ��ͼ3�У���ȷ����A��ѡ����ĸ��
��N2O4��O2��H2O���ϵĻ�ѧ����ʽ��2N2O4+O2+2H2O=4HNO3
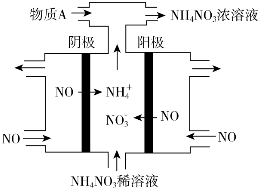
��4�����У����NO�Ʊ�NH4NO3����ԭ��ԭ����ͼ4��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3���貹������A��A��NH3��˵�����ɣ����ݷ�Ӧ8NO+7H2O$\frac{\underline{\;ͨ��\;}}{\;}$3NH4NO3+2HNO3��������ɵ�HNO3�࣮
���� ��1�������������ڴ������ȵ�����������NO��ˮ��
��2������֪2NO��g��+O2��g��?2N02��g���������������С�ķ�Ӧ������ѹǿ��ƽ���Ӱ�������
�ڸ���ͼ��2�жϸ÷�Ӧ�������Ƿ��Ȼ������ȣ����ж�K���¶ȵı仯��
��3���ٽ����¶ȣ���NO2��g��ת��ΪN2O4��l��˵����Ӧ2NO2��g��?N2O4��l��Ϊ���ȷ�Ӧ��ͬ������Һ̬ʱ��������̬ʱ�����ͣ�
��N2O4��O2��H2O�����������ᣬ���ݵ�ʧ�����غ��ԭ���غ�д����Ӧ�ķ���ʽ��
��4�����ݵ��NO�Ʊ�NH4NO3�ķ�Ӧ����ʽ�����жϣ�
��� �⣺��1�������������ڴ������ȵ�����������NO��ˮ����Ӧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��2������֪2NO��g��+O2��g��?2N02��g���������������С�ķ�Ӧ������ѹǿƽ�����ƣ���NO��ת���ʻ�������ͼ��֪P2ʱNO��ת���ʴ���P2ʱѹǿ��
��P1��P2��
�ʴ�Ϊ��P1��P2��
����ͼ��2��֪�������¶ȵ����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ�����ƣ���÷�Ӧ�������Ƿ��ȷ�Ӧ�����������¶�ƽ�ⳣ��K��С��
�ʴ�Ϊ����С��
��3���ٽ����¶ȣ���NO2��g��ת��ΪN2O4��l��˵����Ӧ2NO2��g��?N2O4��l��Ϊ���ȷ�Ӧ��������ͼ���и÷�Ӧ�ķ�Ӧ�������������������������ߣ�ͬ��������̬��Һ̬��ų���������Һ̬ʱ��������̬ʱ�����ͣ���N2O4��l�����е�������N2O4��g�����е������ͣ�ͼ��A���ϣ���A��ȷ��
�ʴ�Ϊ��A��
��N2O4��O2��H2O�����������ᣬ�䷴Ӧ�Ļ�ѧ����ʽΪ��2N2O4+O2+2H2O=4HNO3���ʴ�Ϊ��2N2O4+O2+2H2O=4HNO3��
��4�����NO�Ʊ�NH4NO3��������ӦΪNO-3e-+2H2O=NO3-+4H+��������ӦΪ��NO+5e-+6H+=NH4++H2O����������Ӧ�ɿ�����Ҫʹ��ʧ�����غ㣬����������NO3-�����ʵ�����������������NH4+�����ʵ������ܷ�Ӧ����ʽΪ��8NO+7H2O$\frac{\underline{\;ͨ��\;}}{\;}$3NH4NO3+2HNO3�������Ҫʹ������ȫ��ת��ΪNH4NO3���貹��NH3��
�ʴ�Ϊ��NH3�����ݷ�Ӧ8NO+7H2O$\frac{\underline{\;ͨ��\;}}{\;}$3NH4NO3+2HNO3��������ɵ�HNO3�࣮
���� ���⿼���˻�ѧ����ʽ��д��Ӱ��ƽ�⼰ƽ�ⳣ�������ء������仯ͼ�ķ����ȣ���Ŀ�漰��֪ʶ��϶࣬�����ڿ���ѧ�����ۺ������������Ѷ��еȣ�ע�����֪ʶ�Ļ������գ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | þ���Ͻ�ȿ���ȫ���ڹ��������ֿ���ȫ���ڹ���NaOH��Һ | |
| B�� | �ڼ��������ܷų���������Һ�У�K+��NH4+��CO32-��Cl-һ���ܹ��������� | |
| C�� | �Ȼ�����Һ�м��������ˮ��Ӧ��ʵ�ʣ�Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| D�� | �������ȷ�Ӧԭ�����ܷ�����Ӧ2Al+3MgO$\frac{\underline{\;����\;}}{\;}$3Mg+A12O3 |

| A�� | ����Ԫ���У�����������ˮ�������ԣ�Z����ǿ | |
| B�� | �⻯���ȶ��ԣ�Y����ǿ | |
| C�� | ԭ�Ӱ뾶��С����˳��Y��Z��M��X | |
| D�� | ͬ����Ԫ����MԪ��ԭ��ʧ������࣬�䵥�ʵĻ�ԭ����ǿ |
| A�� | 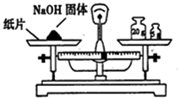 ��ȡNaOH���� | B�� | 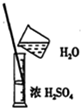 ϡ��Ũ���� | ||
| C�� | 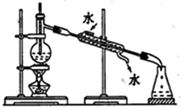 �����Ҵ���ˮ����� | D�� |  �������ͭ |
| A�� | ��⻯������Һ��ͨ�������������2Fe2++2I-+2Cl2=2Fe3++I2+4Cl- | |
| B�� | ������SO2ͨ�뱽������Һ�У�2C6H5O-+SO2+H2O��2C6H5OH+SO32- | |
| C�� | ��FeSO4��ȥ���Է�ˮ�е�Cr2O72-��Cr2O72-+Fe2++14H+�T2Cr3++Fe3++7H2O | |
| D�� | ��ҵ�Ͽ��õ�ⷨ�Ʊ�Mg��2MgO$\frac{\underline{\;����\;}}{���}$2Mg+O2�� |
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ����ɫ��Һ$\stackrel{NaOH��Һ}{��}$���ɫ���� | ˵��ԭ��Һ��һ������FeCl3 |
| B | ��ɫMg��OH��2 $\stackrel{CuSO_{4}��Һ}{��}$��ɫCu��OH��2 | Mg��OH��2���ܶȻ�����Cu��OH��2 |
| C | ���ռ�������$\stackrel{Ba��NO_{3}��_{2}��Һ}{��}$��ɫ���� | ������һ������SO42- |
| D | H3PO3+2HaOH���������TNa2HPO2+2H2O | H3PO3������Ԫ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |