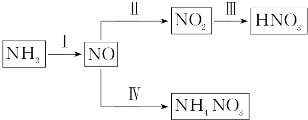
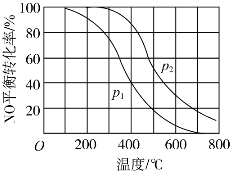
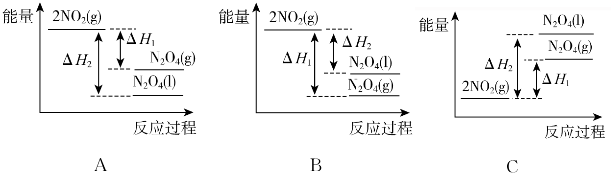
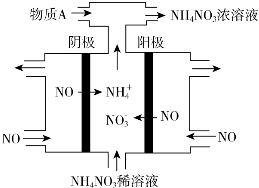
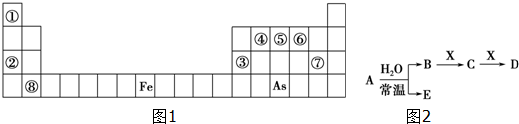
��Ŀ����
1�����н�����ʵ�Ļ�ѧ����ʽ�����ӷ���ʽ��ȷ���ǣ�������| A�� | ��⻯������Һ��ͨ�������������2Fe2++2I-+2Cl2=2Fe3++I2+4Cl- | |
| B�� | ������SO2ͨ�뱽������Һ�У�2C6H5O-+SO2+H2O��2C6H5OH+SO32- | |
| C�� | ��FeSO4��ȥ���Է�ˮ�е�Cr2O72-��Cr2O72-+Fe2++14H+�T2Cr3++Fe3++7H2O | |
| D�� | ��ҵ�Ͽ��õ�ⷨ�Ʊ�Mg��2MgO$\frac{\underline{\;����\;}}{���}$2Mg+O2�� |
���� A���������������������ӡ�������ȫ����������
B����Ӧ���ɱ��Ӻ��������ƣ�
C������������ԭ��Ӧ�����ӡ���ɲ��غ㣻
D����������Ȼ�þұ��Mg����MgO���۵�ߣ�
��� �⣺A����⻯������Һ��ͨ����������������ӷ�ӦΪ2Fe2++4I-+3Cl2=2Fe3++2I2+6Cl-����A����
B������SO2ͨ�뱽������Һ�е����ӷ�ӦΪ2C6H5O-+SO2+H2O��2C6H5OH+SO32-����B��ȷ��
C����FeSO4��ȥ���Է�ˮ�е�Cr2O72-�����ӷ�ӦΪCr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O����C����
D����ҵ�Ͽ��õ�ⷨ�Ʊ�Mg�ķ�ӦΪMgCl2$\frac{\underline{\;����\;}}{���}$2Mg+Cl2������D����
��ѡB��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ�������������ԭ��Ӧ�����ӷ�Ӧ���飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
16�������£�������Һ�е�pH�жϻ����Ũ�ȹ�ϵ������ȷ���ǣ�������
| A�� | ��5mL0.02mol/L��H2SO4��Һ��5mL0.02mol/LNaOH��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ10mL������Һ��pH=2 | |
| B�� | ��0.2mol/L��ijһԪ��HA��Һ��0.1mol/LNaOH��Һ�������ϣ����ҺpH����7����Ӧ��Ļ��Һ�У�c��OH-��+c��A-����c��H+��+c��HA�� | |
| C�� | pH��ȵĢ�CH3COONa ��C6H5ONa ��NaHCO3��Һ�У�c��Na+����С��ϵ���٣��ۣ��� | |
| D�� | �����£�0.1mol/L��CH3COONa��NaClO�Ļ����Һ�У�c��OH-��-c��H+��=c��HClO��+c��CH3COOH�� |
13������Ԫ��X��Y��Z������ΪaX+��bY2-��cZ-���뾶��С��ϵ��X+��Z-��Y2-����˵��������ǣ�������
| A�� | Y2-�Ļ�ԭ�Դ���Z? | B�� | ���ʵĻ�ԭ��Z��Y | ||
| C�� | bһ��С��c | D�� | X��Y�ɴ���ͬ���ڻ�X��Y�������� |
6��������Һ�д��ڵ���ƽ��CH3COOH?H++CH3COO-������������ȷ���ǣ�������
| A�� | CH3COOH��Һ�м�������CH3COONa���壬ƽ�������ƶ� | |
| B�� | 0.10 mol/L��CH3COOH��Һ�м�ˮϡ�ͣ���Һ��c��OH-����С | |
| C�� | ������Һ������Ũ�ȵĹ�ϵ���㣺c��H+��=c��OH-��+c��CH3COO-�� | |
| D�� | ������pH=2��CH3COOH��Һ��pH=12��NaOH��Һ�������Ϻ���Һ��pH��7 |

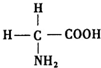 �������ǵ����ʵĻ�ʯ���ʰ�������İ����ᣨ�ṹ��ͼ�����ڸʰ�������У�Nԭ�ӵ��ӻ���ʽ��sp3���ڸʰ��������C��N��Oԭ�ӵĵ�һ�����ܴӴ�С��˳����N��O��C���������γɶ������ӣ���N3-��NH2-��NH4+��N2H5+�ȣ����У�NH4+�Ŀռ乹�����������壮
�������ǵ����ʵĻ�ʯ���ʰ�������İ����ᣨ�ṹ��ͼ�����ڸʰ�������У�Nԭ�ӵ��ӻ���ʽ��sp3���ڸʰ��������C��N��Oԭ�ӵĵ�һ�����ܴӴ�С��˳����N��O��C���������γɶ������ӣ���N3-��NH2-��NH4+��N2H5+�ȣ����У�NH4+�Ŀռ乹�����������壮