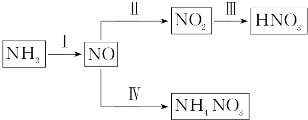
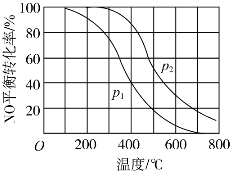
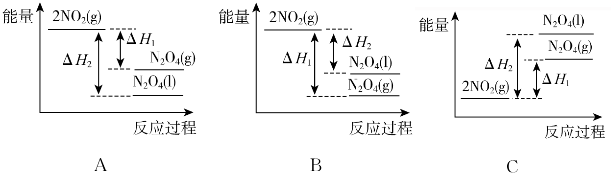
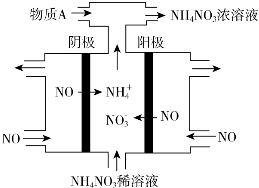
��Ŀ����
4��Ԫ�����ڱ��е�Ԫ��λ����ͼ��ʾ��
��ش��������⣺
��1���ɢڢܢ�����Ԫ����ɵ������Է���������106������ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��CO32-+H2O?HCO3-+OH-��
��2��ֻ���٢ۢ�����Ԫ�ص�һ�ֳ������ӻ������У�Ԫ�آ۵Ļ��ϼ۷ֱ�����ۺ���ͼۣ���ˮ��Һ�����ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ��c��N03-����c��NH4+����c��H+����c��OH-����
��3��д��Ԫ�آߵĵ�����Ԫ�آݵ�����������ˮ�����ˮ��Һ��Ӧ�����ӷ���ʽ��Si+2OH-+H2O=SiO32-+2H2����
��4����Ԫ�آ���Ԫ�آ�ĸۻ���������ˮ�У����ӷ���ʽ�ǣ�Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3H+��
��5��д����Ԫ�آ�ĵ���Ϊ�缫������ɢܢݢ���ɵĻ������Է���������142����ˮ��Һ���ܻ�ѧ����ʽ��Fe+2H2O$\frac{\underline{\;���\;}}{\;}$Fe��OH��2��+H2����
��6����Ԫ�آܢ���ɵ������ӿ���С�մ���Һ��Ӧ�������ӷ���ʽΪ��AlO2-+HCO3-+H20=Al��OH��3��+CO32-��
��7���ɢ٣�X���ڣ�Y���ܣ�Z������Ԫ�صĻ���������ʽ�������ǣ�D��
A��X2YZ��B��X2YZ2��C��X2YZ3��D��X2YZ4��E��X2Y2Z4��
��8��Ԫ�آ۵��⻯����Ԫ�آܵĵ�����һ�������·����û���Ӧ���ڸ÷�Ӧ�У��������ͻ�ԭ�������ʵ���֮��Ϊ��3��4��
���� ����Ԫ�������ڱ��еķֲ���֪�١���Ԫ�طֱ��ǣ�H��C��N��O��Na��Al��Si��S��Cl��Fe��
��1���ڢܢ�����Ԫ�طֱ�ΪC��O��Na����C��O��Na��ɵ������Է���������106��Ϊ̼���ƣ�̼������Ӳ���ˮ�⣬��Һ��ʾ���ԣ��ݴ�д��̼�������ˮ������ӷ���ʽ��
��2���û�����Ϊ����泥�笠����Ӳ���ˮ�⣬��Һ��ʾ���ԣ�Ȼ���ϵ���غ��жϸ�����Ũ�ȴ�С��
��3��Ԫ�آߵĵ���ΪSi��Ԫ�آݵ�����������ˮ����ΪNaOH����������������Һ��Ӧ���ɹ����ƺ�������
��4��Ԫ�آ�ΪCl��Ԫ�آ�ĸ�����Ϊ�����ӣ������γɵĻ�����Ϊ�Ȼ��������Ȼ��������ˮ�У������ӷ���ˮ�����������������壻
��5���آ�ĵ���FeΪ�缫���ɢܢݢ���ɵĻ������Է���������142�������ƣ������������ʧȥ������������������������ˮ����������ӵõ����������������ݴ�д�������ܷ���ʽ��
��6����Ԫ�آܢ���ɵ������ӿ���С�մ���Һ��Ӧ����������Ϊƫ��������ӣ�̼��������ӵ����Դ���������������̼�����������ƫ��������ӷ�Ӧ������������������̼������ӣ�
��7���٣�X��ΪH���ڣ�Y��ΪC���ܣ�Z��ΪOԪ�أ���H��C��O����Ԫ�صĻ����H���ϼ�Ϊ+1��OԪ�ػ��ϼ�Ϊ-2�����̼Ԫ�ص��������Ϊ+4�Ը�ѡ������жϣ�
��8��Ԫ�آ۵��⻯��Ϊ������Ԫ�آܵĵ���Ϊ��������һ�������·����û���Ӧ����Ӧ���ɵ�����ˮ������������ԭ��Ӧ�е����غ���㣮
��� �⣺����Ԫ�������ڱ��еķֲ���֪�١���Ԫ�طֱ��ǣ�H��C��N��O��Na��Al��Si��S��Cl��Fe��
��1���ڢܢݷֱ�ΪC��O��Na����C��O��Na��ɵ��������Է�������Ϊ106���������Ϊ̼���ƣ�̼������Һ��̼������Ӳ���ˮ�⣬��Һ��ʾ���ԣ�ˮ������ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-����
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��2���٢ֱܷۢ�ΪH��N��O��ֻ���٢ۢ�����Ԫ�ص�һ�ֳ������ӻ������У�Ԫ�آ۵Ļ��ϼ۷ֱ�����ۺ���ͼۣ��û�����Ϊ����泥�笠����Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��H+����c��OH-�������ݵ���غ��֪��c��N03-����c ��NH4+��������Һ������Ũ�ȴ�СΪ��c��N03-����c ��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��N03-����c ��NH4+����c��H+����c��OH-����
��3��Ԫ�آߵĵ���ΪSi��Ԫ�آݵ�����������ˮ����ΪNaOH����������������Һ��Ӧ���ɹ����ƺ���������Ӧ�����ӷ���ʽΪ��Si+2OH-+H2O=SiO32-+2H2����
�ʴ�Ϊ��Si+2OH-+H2O=SiO32-+2H2����
��4��Ԫ�آ���Ԫ�آ�ĸۻ�����Ϊ�Ȼ��������Ȼ��������ˮ�У������ӷ���ˮ�����������������壬��Ӧ�����ӷ���ʽΪ��Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3H+��
�ʴ�Ϊ��Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3H+��
��5��Ԫ�آ�ĵ���FeΪ�缫���ɢܢݢ���ɵĻ������Է���������142�������ƣ��������У�������ʧȥ������������������������ˮ����������ӵõ��������������������ܻ�ѧ����ʽΪ��Fe+2H2O $\frac{\underline{\;���\;}}{\;}$ Fe��OH��2��+H2����
�ʴ�Ϊ��Fe+2H2O $\frac{\underline{\;���\;}}{\;}$ Fe��OH��2��+H2����
��6����Ԫ�آܢ���ɵ������ӿ���С�մ���Һ��Ӧ����������Ϊƫ��������ӣ�����̼��������ӵ����Դ���������������̼�����������ƫ��������ӷ�Ӧ��������������������Ӧ�����ӷ���ʽΪ��AlO2-+HCO3-+H20=Al��OH��3��+CO32-��
�ʴ�Ϊ��AlO2-+HCO3-+H20=Al��OH��3��+CO32-��
��7���٣�X��ΪH���ڣ�Y��ΪC���ܣ�Z��ΪOԪ�أ���H��C��O����Ԫ�صĻ����H���ϼ�Ϊ+1��OԪ�ػ��ϼ�Ϊ-2��CԪ�ػ��ϼۿ���Ϊ
A��X2YZΪH2CO��Ϊ��ȩ�����Դ��ڸ����ʣ���A����
B��X2YZ2ΪH2CO2��Ϊ���ᣬ���ڸ����ʣ���B����
C��X2YZ3ΪH2CO3��Ϊ̼�ᣬ���ڸ����ʣ���C����
D��X2YZ4ΪH2CO4��CԪ�صĻ��ϼ�Ϊ+6����C������ϼ�Ϊ+4�����Բ����ڸ����ʣ���D��ȷ��
E��X2Y2Z4ΪH2C2O4��Ϊ���ᣬ���ϼ�Ϊ+2�����ڸ����ʣ���E����
�ʴ�Ϊ��D��
��8��Ԫ�آ۵��⻯��Ϊ������Ԫ�آܵĵ���Ϊ��������һ�������·����û���Ӧ����Ӧ����Ϊ������ˮ�������е�Ԫ�صĻ��ϼ�Ϊ-3����Ӧ������0�۵ĵ��������ϼ۱仯Ϊ3����Ӧ��NԪ�ػ��ϼ����߱�����������Ϊ��ԭ����������Ӧ�����ɱ��-2�ۣ����ϼ۱仯Ϊ4�������������������ʵ���Ϊx����ԭ�����������ʵ���Ϊy�����ݵ����غ�ɵã�4x=3y�������ɵã�x��y=3��4��
�ʴ�Ϊ��3��4��
���� ���⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������������Ӧ�û���֪ʶ����������������Ԫ�����������ݡ�Ԫ�����ڱ��ṹΪ���ؼ���

| A�� | NaOH��Һ | B�� | ��ˮ | C�� | AgNO3��Һ | D�� | NaCl��Һ |
| A�� | pH=5.6��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� | |
| B�� | ��pH=a�Ĵ���ϡ��ΪpH=a+1�Ĺ����У�$\frac{{cCH}_{3}COOH}{{cH}^{+}}$��С | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��CH3COOH��CH3COONa��Һ�������Ϻ�c��CH3COO-��-c��CH3COOH��=2c��H+��-c��OH-�� | |
| D�� |  ��10.00 mL 0.1 mol•L-1HCOOH��Һ����μ���0.1 mol•L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯������c��NaOH��Һ�����С��10 mL ��10.00 mL 0.1 mol•L-1HCOOH��Һ����μ���0.1 mol•L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯������c��NaOH��Һ�����С��10 mL |
| A�� | ����ˮ�����Һ©�����������Ҵ������ã��ɽ�����ȡ���Ҵ��� | |
| B�� | ���Ȼ���������Һ��У�����ȡ������������ | |
| C�� | ij��ɫ��Һ�м�������ϡ���������������ٵμ�BaCl2��Һ�а�ɫ�������ɣ���ԭ��Һ��һ������SO42- | |
| D�� | ��������˿պȡij��Һ�ھƾ��������գ�����ʻ�ɫ����˵������Һ��һ������������ |
| A�� | ��5mL0.02mol/L��H2SO4��Һ��5mL0.02mol/LNaOH��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ10mL������Һ��pH=2 | |
| B�� | ��0.2mol/L��ijһԪ��HA��Һ��0.1mol/LNaOH��Һ�������ϣ����ҺpH����7����Ӧ��Ļ��Һ�У�c��OH-��+c��A-����c��H+��+c��HA�� | |
| C�� | pH��ȵĢ�CH3COONa ��C6H5ONa ��NaHCO3��Һ�У�c��Na+����С��ϵ���٣��ۣ��� | |
| D�� | �����£�0.1mol/L��CH3COONa��NaClO�Ļ����Һ�У�c��OH-��-c��H+��=c��HClO��+c��CH3COOH�� |
| A�� | Y2-�Ļ�ԭ�Դ���Z? | B�� | ���ʵĻ�ԭ��Z��Y | ||
| C�� | bһ��С��c | D�� | X��Y�ɴ���ͬ���ڻ�X��Y�������� |
 ij��Һֻ�����������ӣ�NH4+��Na+��Fe2+��NO3-��I-��SO32-��AlO2-�е����֣�����ˮ�ĵ��룩���Ҹ����ӵ����ʵ���Ũ����ȣ��ֽ�������ʵ�飺
ij��Һֻ�����������ӣ�NH4+��Na+��Fe2+��NO3-��I-��SO32-��AlO2-�е����֣�����ˮ�ĵ��룩���Ҹ����ӵ����ʵ���Ũ����ȣ��ֽ�������ʵ�飺��ȡ��������Һ��NaOH��Һ���ȣ������̼�����ζ�����壬δ�۲쵽������������
����ȡ��������Һ�����������ᣬ�ɹ۲쵽����ɫ���壬δ�۲쵽�������ɣ�
��������ʵ�飬����˵����ȷ���ǣ�������
| A�� | ��ʵ���ֻ��ȷ��ԭ��Һ��һ����NH4+��û��Fe2+�� | |
| B�� | ȡʵ��ں����Һ�μӵ�����Һ�����ܱ���ɫ�� | |
| C�� | ԭ��Һ�п��ܺ���NH4+��Na+��SO32-��I-�������� | |
| D�� | ȡ����ԭ��Һ�����Ը��������Һ���������������ӱ����� |
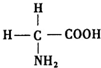 �������ǵ����ʵĻ�ʯ���ʰ�������İ����ᣨ�ṹ��ͼ�����ڸʰ�������У�Nԭ�ӵ��ӻ���ʽ��sp3���ڸʰ��������C��N��Oԭ�ӵĵ�һ�����ܴӴ�С��˳����N��O��C���������γɶ������ӣ���N3-��NH2-��NH4+��N2H5+�ȣ����У�NH4+�Ŀռ乹�����������壮
�������ǵ����ʵĻ�ʯ���ʰ�������İ����ᣨ�ṹ��ͼ�����ڸʰ�������У�Nԭ�ӵ��ӻ���ʽ��sp3���ڸʰ��������C��N��Oԭ�ӵĵ�һ�����ܴӴ�С��˳����N��O��C���������γɶ������ӣ���N3-��NH2-��NH4+��N2H5+�ȣ����У�NH4+�Ŀռ乹�����������壮