��Ŀ����
3����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
��1��װ��A�������ķ���װ�ã�����ʢ��Ũ��������������Ƿ�Һ©������д���÷�Ӧ��Ӧ�Ļ�ѧ����ʽ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��ͬʱװ��BҲ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����B�г���©����Һ���������γ�ˮ����
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ����Ӧ��d������ĸ��ţ���
| ��� | �� | �� | �� |
| a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
| c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��5��װ��F�����������ն������������ֹ��Ⱦ���������ձ��е���Һ ����ѡ�������е�b������ĸ��ţ���
a������NaOH��Һ
b������Ca��OH��2��Һ
c������Na2SO3��Һ
d������Na2CO3��Һ��
���� ��1��������������״�ж����������ƣ�������ƾ���ǿ�������ԣ��ܹ�����Ũ���������������Ȼ��ƺ�ˮ��
��2����װ��A����ȡ�������к����Ȼ������ʣ��Ȼ���������ˮ�������ڱ���ʳ��ˮ���ܽ�Ȳ���
��C�ж�������B��ѹǿ�����ݴ˷�����������
��3����֤�����Ƿ���Ư���ԣ��������ø�����ɫ������ʪ�����ɫ���������Ա�ʵ�飻
��4�������������ԡ����������ǿ�ڵⵥ�ʣ��ܹ��������������ɵ��ʵ⣬���ʵ������ڱ������ܶ�С��ˮ�ܶȣ�
����������Ҳ�ܹ��������������ɵ��ʵ⣻
��5�������ж���ֱ���ŷ��ܹ����������Ⱦ��Ӧ����β��������
��������Ϊ�������Ca��OH��2��Һ�����������ӽ��ٲ�����ȫ����ʣ���������
��� �⣺��1��ͼ��ʢ��Ũ���������Ϊ��Һ©����
���������Ũ�����ڳ����·�Ӧ�����Ȼ��ơ�������ˮ����ѧ����ʽΪ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O��
�ʴ�Ϊ����Һ©����Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O��
��2������Ũ������лӷ��ԣ���Aװ�ó����������к����Ȼ������壬�Ȼ���������ˮ�������ڱ���ʳ��ˮ���ܽ�Ȳ�����ͨ������ʳ��ˮ��ȥ��
��C�ж�������B��ѹǿ������ˮ��©����ѹ���Ӷ��γ�һ��ˮ����
�ʴ�Ϊ����ȥCl2�е�HCl��B�г���©����Һ���������γ�ˮ����
��3��Ҫ��֤�����Ƿ���Ư���ԣ�����������ɫ��������Ϊ��ȡ����������ˮ���������ɵ�HClO��Ư���ԣ�����֤�����Ƿ��ܹ�ʹʪ�����ɫ������ɫ��Ȼ���ø�������ͨ���������ɫ��������������Ư���ԣ�������������ѡ����������Ӧ�ļ�ʯ�ң�U�ι�һ��ʢװ����������Ũ�����ʢװ��U�ι��У���ˮ����ͭ���Լ���ˮ�Ĵ��ڣ���������������������ѡ����ˮ�Ȼ��ƣ�������ȷ��ѡ����d��
�ʴ�Ϊ��d��
��4���������֪������ͨ��D�е��廯�������У��û����嵥�ʣ��嵥�ʵ�������ǿ�ڵⵥ�ʣ��������ڱ����Ϻ�ɫ�����ܶ�С��ˮ�����Կ�������E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
����D�п����й���������������Ҳ���������������ɵ⣬��E�����ɵĵ��ʵⲻһ��������KI��Ӧ�û������ģ�Ҳ�����ǹ�����Cl2Ҳ�ɽ�I-����ΪI2���ʲ���˵���嵥�ʵ�������һ���ȵ�ǿ��
�ʴ�Ϊ��E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ�����ܣ�������Cl2Ҳ���Խ�I-����ΪI2��
��5�������ж���ֱ���ŷ��ܹ����������Ⱦ��Ӧ����β��������װ��F�������ǣ����ն������������ֹ��Ⱦ������
a��NaOH��Һ��������Ӧ�����Ȼ��ơ��������ƺ�ˮ���ܹ����չ�������������ȷ��
b����������Ϊ�������Ca��OH��2��Һ�����������ӽ��٣�����Ca��OH��2��Һ������������֣��ʴ���
c���������ƾ���ǿ�Ļ�ԭ�ԣ��ܹ�����������������ԭ��Ӧ����������������ȷ��
d����������ˮ��Ӧ�������ᣬ�����뱥��̼������Һ��Ӧ���ܹ��ñ���̼������Һ���գ�����ȷ��
�ʴ�Ϊ�����ն������������ֹ��Ⱦ������b��
���� ���⿼�����������Ʊ������ʵļ��飬��Ϥ�������Ʊ�ԭ���������Ļ�ѧ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ����̬�⻯����ȶ��� | |
| B�� | ���ʵ������Ե�ǿ�� | |
| C�� | ������������Ӧ��ˮ���������� | |
| D�� | ���ʵ��۵�ߵ� |
| A�� | ��ˮ��ͨ��������Cl2+H2O�TH++Cl-+HClO | |
| B�� | ��������Һ�мӹ�����ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O | |
| C�� | ����������Һ����μ�������������Һ������Һǡ�ó����ԣ�Ba2++OHһ+SO42-+H+�TBaSO4��+H2O | |
| D�� | ��������6mol/L��������ˮ��Һ���ȣ�C2H5Br+OH-CH2�TCH2��+Br-+H2O |
| A�� | 3��2��1 | B�� | 2��6��3 | C�� | 3��6��2 | D�� | 2��1��3 |
 һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ���ǣ�������
һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ���ǣ�������| A�� | ��Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ0.158mol/��L•s�� | |
| B�� | ��Ӧ��ʼ��10s��X�����ʵ���Ũ�ȼ�����0.79mol/L | |
| C�� | ��Ӧ��ʼ��10sʱ��Y��ת����Ϊ79.0% | |
| D�� | ��Ӧ�Ļ�ѧ����ʽΪ��2X��g��+2Y��g��?Z��g�� |
| A�� | Na2O2�ĵ���ʽΪ | |
| B�� | ԭ������������Ϊ2��Ԫ��һ��λ�����ڱ��ڢ�A�� | |
| C�� | �ڵ�ԭ���У�������Ϊ7����������һ��Ϊ7 | |
| D�� | Cl-�Ľṹʾ��ͼΪ  |
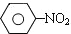 $\stackrel{Fe��HCl��H_{2}O}{��}$
$\stackrel{Fe��HCl��H_{2}O}{��}$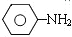 �������������ԡ��ױ�������
�������������ԡ��ױ������� $\stackrel{KMnO_{4}��H+}{��}$
$\stackrel{KMnO_{4}��H+}{��}$
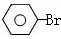 $��_{���¡���ѹ}^{NaOH��Һ������}$
$��_{���¡���ѹ}^{NaOH��Һ������}$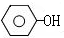
 ������ˮ�����ƣ�
������ˮ�����ƣ�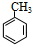 +3HO-NO2$��_{��}^{Ũ����}$
+3HO-NO2$��_{��}^{Ũ����}$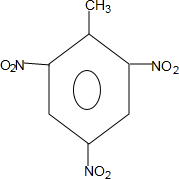 +3H2O
+3H2O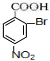 D��
D��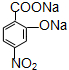 ��
��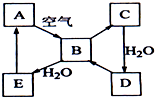 ��ͼ��ʾ����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ�
��ͼ��ʾ����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ�