��Ŀ����
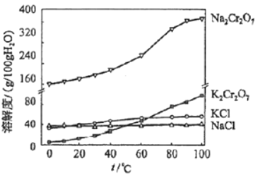
����Ŀ�������ѿ�̫���ܵ�ؾ߱�������ࡢ����Ӧ�á�����ɱ��ͺ�Ч�ʸߵ������ŵ㡣 һ�ָ��ѿ�̫���ܵ�ز��ϵľ�����ͼ��ʾ��
�밴Ҫ��ش������й����⣺
��1������ CH3NH3 ������Ԫ���е縺����С����_____________�� д��̼ԭ�ӵĵ����Ų�ʽΪ__________��
��2��Pb �� C ͬ���壬�� C ������������ 4��д�� Pb ԭ���������ӵĹ����ʾʽ���������Ų�ͼ��__________________��
��3���й� NH3 �Ľṹ������̽��
�� NH3 ������������ѧ����____________���������Լ��������Ǽ��Լ���������λ������ �����Ӽ����������������� ��������ѡ����ϵ����ƣ���N ԭ�ӵ��ӻ���ʽΪ__________��
�� NH3 ���ӵ� VSEPR ģ������Ϊ_________�����ӵĿռ�ṹ�������幹�ͣ� Ϊ______��
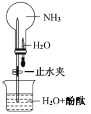
����ͼ��ʾ��̽�� NH3 ������ʱ����ֹˮ�У���ѹ��ͷ�ιܣ����Թ۲쵽��ƿ��Ѹ�ٲ�����ɫ��Ȫ�����ñ�Ҫ�ķ��ӽṹ �����ʵ�֪ʶ�ͻ�ѧ������Ͳ����������ԭ��_________��________��
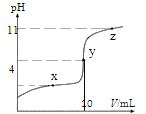
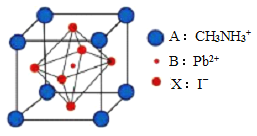
��4���ø��ѿ�̫���ܵ�ز��ϵĻ�ѧʽΪ________________���þ����У��� I- ���ڵ� I- ����Ϊ_____________�� �ⶨ�侧��ṹ������ɿ��ķ�����____________ʵ�顣
���𰸡�H 1s22s22p2 ![]() ���Լ������� sp3 ������ ������ NH3����Ϊ���Է��ӣ�����NH3������H2O���Ӽ��γ�������ʰ�����������ˮ��ʹ��ƿ����ѹ��С���γ���Ȫ NH3��H2O��Ӧ����NH3��H2O ��NH3��H2O
���Լ������� sp3 ������ ������ NH3����Ϊ���Է��ӣ�����NH3������H2O���Ӽ��γ�������ʰ�����������ˮ��ʹ��ƿ����ѹ��С���γ���Ȫ NH3��H2O��Ӧ����NH3��H2O ��NH3��H2O![]() NH4++OH-����ˮ�ʼ��ԣ���ˮ��ʹ��̪��Һ��죬�����γɺ�ɫ��Ȫ PbCH3NH3I3 8 X-��������
NH4++OH-����ˮ�ʼ��ԣ���ˮ��ʹ��̪��Һ��죬�����γɺ�ɫ��Ȫ PbCH3NH3I3 8 X-��������
��������
��1�����鳣��Ԫ��H��C��N�縺�ԵĴ�С��C�ĺ˵����Ϊ6��CԪ�صĺ�������Ų�ʽ��
��2��ͬ����Ԫ�أ��۵�������ͬ���۵����Ų�ʽ���ƣ���Pbԭ�����������Ų�ʽΪ6s26p2��Pbԭ���������ӵĹ��ʽ��![]() ��
��
��3�����ü۲���Ӷ���ȷ��NH3���ӵ�����ԭ��N���ӻ���ʽ��NH3���ӵ�VSEPRģ������Ϊ�������Σ������Ƿ��йµ��Ӷԣ����м����µ��Ӷԣ�ȷ�����ӵĿռ乹�ͣ�NH3���ӵļ۲���Ӷ�����4��Nԭ�ӵ��ӻ���ʽΪsp3��VSEPRģ������Ϊ�������Σ�����ΪNԭ������һ�Թµ��Ӷԣ����ӵĿռ�ṹ(�����幹��)Ϊ�����Σ�NH3����Ϊ���Է��ӣ�����NH3������H2O���Ӽ��γ������������������ˮ��ʹ��ƿ����ѹ��С���γ���Ȫ����ˮ�ʼ��ԣ��ʷ�̪�Լ�������ˮ�Ժ�ɫ��
��4�����ݾ����ṹͼ����ȷ��һ�������к��ж��ٸ�A��B��X���ⶨ����ṹ������ɿ��ķ�����X-��������ʵ�顣
��1��H��C��N����Ԫ�ص縺�ԵĴ�С˳���ǣ�N>C>H��������Ԫ���е縺����С����H��C�˵������6��̼ԭ�ӵĵ����Ų�ʽΪ��1s22s22p2��
��2��C������������2��Pb������������C������������4����Pb����������Ϊ6��Pbԭ�����������Ų�ʽΪ6s26p2��Pbԭ���������ӵĹ��ʽ��![]() ��
��
��3���� NH3������N��H֮���γɵĻ�ѧ��N-H�Ǽ��Թ��ۼ���������1��NH3��������3��������1�Թµ��Ӷԣ��۲���Ӷ���Ϊ4��Nԭ�ӵ��ӻ���ʽΪsp3��
�� NH3���ӵ�VSEPRģ������Ϊ�������Σ����ӵĿռ�ṹ(�����幹��)Ϊ�����Σ�
��NH3����Ϊ���Է��ӣ�����NH3������H2O���Ӽ��γ�������ʰ�����������ˮ��ʹ��ƿ����ѹ��С���γ���Ȫ��NH3��H2O��Ӧ����NH3��H2O ��NH3��H2O![]() NH4++OH-����ˮ�ʼ��ԣ���ˮ��ʹ��̪��Һ��죬�����γɺ�ɫ��Ȫ��
NH4++OH-����ˮ�ʼ��ԣ���ˮ��ʹ��̪��Һ��죬�����γɺ�ɫ��Ȫ��
��4��8��A�ھ����Ķ��㣬1��B�����ģ�6��X�����ģ��ʾ����к���A�ĸ�����8��![]() =1�������к���B�ĸ�����1�������к���X�ĸ�����6��
=1�������к���B�ĸ�����1�������к���X�ĸ�����6��![]() =3���ø��ѿ�̫���ܵ�ز��ϵĻ�ѧʽΪPbCH3NH3I3���þ����У���I- ���ڵ� I-����Ϊ8���ⶨ����ṹ������ɿ��ķ�����X-��������ʵ�顣
=3���ø��ѿ�̫���ܵ�ز��ϵĻ�ѧʽΪPbCH3NH3I3���þ����У���I- ���ڵ� I-����Ϊ8���ⶨ����ṹ������ɿ��ķ�����X-��������ʵ�顣