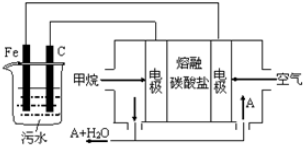
��Ŀ����
3������ѪҺ��Ca2+��Ũ��һ�����g/cm3����ʾ����1cm3Ѫ���к��е�Ca2+������������ȡһ�������Ѫ�����������IJ����[��NH4��2C2O4]��Һ������������ƣ�CaC2O4�����������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵ�������ᣨH2C2O4��������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�
������1������KMnO4����Һ��
��ͼ������100mLKMnO4����Һ�Ĺ���ʾ��ͼ��
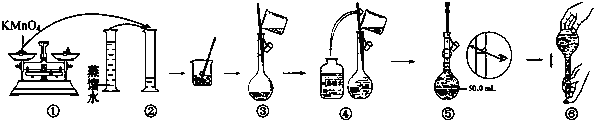
��1������۲�ͼʾ�жϣ����в���ȷ����������Тڢݣ�����ţ���
��2������ȷ��100mL��Һ�����������100mL����ƿ
��3�������ͼʾ�IJ��������Ƶ���Һ����ʵ�飬������������ȷ������£������Ƶ���ҺŨ�Ƚ�ƫС���ƫ��ƫС������
������2���ⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ�
��ȡѪ��20.00mL����������������õ����ᣬ����0.020mol/L ����KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ�����ʱ������12.00mL KMnO4��Һ��
��4��д������������KMnO4��Һ��Ӧ�����ӷ���ʽ2MnO4-+5H2C2O4+6H+�T2 Mn2++10CO2��+8H2O��
��5���ζ����յ�Ϊ�������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��6���������㣬ѪҺ��Ʒ��Ca2+��Ũ��Ϊ1.2��10-3g/cm3��
���� ��1����Ͳֻ����ȡ��Һ������ϡ�ͻ��ܽ�ҩƷ������ʱ������Ҫ�밼Һ����ʹ����У�
��2������100mL��Һ��Ҫ100mL����ƿ��
��3������c=$\frac{n}{V}$�ж������Vƫ���nƫС����������ҺŨ��ƫС�����nƫ���VƫС����������ҺŨ��ƫ��
��4��������л�ԭ�ԣ�������ؾ���ǿ�����ԣ����߷���������ԭ��Ӧ���������ӡ�������̼��ˮ��
��5���ζ��յ�Ϊ���������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��6���õ���ϵʽΪ5Ca2+----5C2O42-----2MnO4-�����ݸ����Ӻ����������֮���ϵʽ���㣮
��� �⣺��1����Ͳֻ����ȡ��Һ������ϡ�ͻ��ܽ�ҩƷ������ʱ������Ҫ�밼Һ����ʹ����У����Դ���Ϊ�ڢݣ��ʴ�Ϊ���ڢݣ�
��2������100mL��Һ��Ҫ100mL����ƿ������ȷ��100mL��Һ�����������100mL����ƿ���ʴ�Ϊ��100 mL����ƿ��
��3������c=$\frac{n}{V}$�ж������Vƫ���nƫС����������ҺŨ��ƫС�����nƫ���VƫС����������ҺŨ��ƫ������ʱ��Һ���ƫ����������ҺŨ��ƫС���ʴ�Ϊ��ƫС��
��4��������л�ԭ�ԣ�������ؾ���ǿ�����ԣ����߷���������ԭ��Ӧ���������ӡ�������̼��ˮ�����ӷ���ʽΪ2MnO4-+5H2C2O4+6H+�T2 Mn2++10CO2��+8H2O���ʴ�Ϊ��2MnO4-+5H2C2O4+6H+�T2 Mn2++10CO2��+8H2O��
��5���ζ��յ�Ϊ���������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ���������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��6���õ���ϵʽΪ5Ca2+----5C2O42-----2MnO4-��������ӵ�����Ϊx��
5Ca2+----5C2O42-----2MnO4-��
200g 2mol
x 0.020mol/L��0.012mL
x=$\frac{200g��0.020mol/L��0.012L}{2mol}$=0.024g��
������Ũ��=$\frac{0.024g}{20mL}$=1.2��10-3g/mL��
�ʴ�Ϊ��1.2��10-3��
���� ���⿼�����ʺ����ⶨ��һ�����ʵ���Ũ����Һ���ƣ�Ϊ��Ƶ���㣬��ȷʵ��ԭ���������淶���ǽⱾ��ؼ����״��������������ѵ��Ǹ����Ӻ����������֮���ϵʽ��ȷ������ĿŨ�Ȳ���

| A�� | ����������6mol/L������ | B�� | ���������Ȼ�ͭ��Һ | ||
| C�� | ������������ˮ | D�� | �����������Ȼ�����Һ |
| A�� | X��Y����ͬһ���� | B�� | ԭ��������a��b | ||
| C�� | a-b=m+n | D�� | ���Ӱ뾶��Xn+��Ym- |
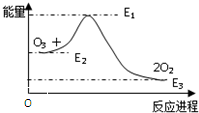 ����������ķ�Ӧ�ǣ�O+O3�T2O2��H���÷�Ӧ�������仯��ͼ��ʾ�����������У���ȷ��������
����������ķ�Ӧ�ǣ�O+O3�T2O2��H���÷�Ӧ�������仯��ͼ��ʾ�����������У���ȷ��������| A�� | O+O3�T2O2�����ȷ�Ӧ | B�� | O+O3�T2O2�Ƿ��ȷ�Ӧ | ||
| C�� | ��ӦO+O3�T2O2�ġ�H=E3-E2 | D�� | ��ӦO+O3�T2O2�ġ�H=E3-E1 |
| A�� | 0.2 mol | B�� | 0.3 mol | C�� | 0.4 mol | D�� | 0.5 mol |
| A�� | ����п�̵���У�MnO2�Ǵ��� | |
| B�� | ͭпԭ��ع���ʱ��Zn������ΪZn2+ | |
| C�� | �ŵ�ʱ��Ǧ������������Ũ�Ȳ������� | |
| D�� | ����ȼ�ϵ����У�ͨ������һ��Ϊ���� |
 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�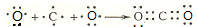 ��
�� ��
��