��Ŀ����
10��Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����ã��±��г��ˢ١������Ԫ�������ڱ��е�λ�ã���ش�| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� |
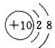 ��
����2������Ԫ���зǽ�������ǿ����F����Ԫ�ط��ţ���Ԫ�آ���⻯��ĵ���ʽΪ
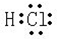 ��
����3�������⣬ԭ�Ӱ뾶������Na����Ԫ�ط��ţ����ڢ١��ڡ�������Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����NaOH���ѧʽ��������ʽΪ
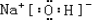 �������еĻ�ѧ�����������Ӽ������Թ��ۼ�������ѡ�����Ӽ����������Թ��ۼ��������Ǽ��Թ��ۼ����е�һ������д����
�������еĻ�ѧ�����������Ӽ������Թ��ۼ�������ѡ�����Ӽ����������Թ��ۼ��������Ǽ��Թ��ۼ����е�һ������д������4���ޢߢ�����Ԫ�ص���̬�⻯����ȶ�����ǿ������˳����HF��HCl��H2S��������Ӧ���⻯��Ļ�ѧʽ����
��5��Ԫ�آܶ�Ӧ���⻯��Ļ�ѧʽ��NH3������ʽΪ
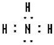 ����ˮ��Һ��pH��7���������������
����ˮ��Һ��pH��7�����������������6���ߢ�����Ԫ�ض�Ӧ����ۺ������������ǿ������˳����HClO4��H2SO4��������Ӧ������������Ӧˮ����Ļ�ѧʽ����
��7���ٵ���������������ӵĵ��Ӳ�ṹ���Ԫ�ص�ԭ�ӵĵ��Ӳ�ṹ��ͬ�����������ӵİ뾶�ɴ�С��˳����F-��Na+������Ӧ���ӷ������𣩣�
��8����ЩԪ�ص�����������Ӧˮ��������Ե���������������������Һ��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
���� ��Ԫ�������ڱ���λ�ÿ�֪������Na������Mg������Al������N������O������F������S������Cl������Ne��
��1������Ԫ����ֻ��Ne�������Ϊ8�����ȶ��ṹ��
��2������Ԫ����F�ĵõ���������ǿ��Ԫ�آ���⻯��ΪHCl��Ϊ���ۻ����
��3�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����ԭ������С��ԭ�Ӱ뾶������Խǿ������������Ӧ��ˮ�������Խǿ��
��4���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��5���ܶ�Ӧ���⻯��ΪNH3��Ϊ���ۻ������Һˮ����һˮ�ϰ������������ֻ��OH-��
��6���ǽ�����Խǿ����Ӧ����ۺ����������Խǿ��
��7��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��
��8�������Ե���������Ϊ������������NaOH��Ӧ����ƫ�����ƺ�ˮ��
��� �⣺��Ԫ�������ڱ���λ�ÿ�֪������Na������Mg������Al������N������O������F������S������Cl������Ne��
��1������Ԫ����ֻ��Ne�������Ϊ8�����ȶ��ṹ����Ne�Ļ�ѧ�������ȶ�����ԭ�ӽṹʾ��ͼΪ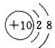 ���ʴ�Ϊ��Ne
���ʴ�Ϊ��Ne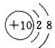 ��
��
��2������Ԫ����F�ĵõ���������ǿ����F�ķǽ�������ǿ��Ԫ�آ���⻯��ΪHCl��Ϊ���ۻ���������ʽΪ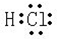 ���ʴ�Ϊ��F��
���ʴ�Ϊ��F��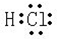 ��
��
��3�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����ԭ������С��ԭ�Ӱ뾶����ԭ�Ӱ뾶����ΪNa��Na�Ľ�������ǿ��������������Ӧ��ˮ���������ǿ���ü�ΪNaOH�������Ӽ���O-H���ۼ��������ʽΪ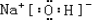 ���ʴ�Ϊ��Na��NaOH��
���ʴ�Ϊ��Na��NaOH��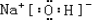 �����Ӽ������Թ��ۼ���
�����Ӽ������Թ��ۼ���
��4���ǽ�����ΪF��Cl��S����̬�⻯����ȶ���ΪHF��HCl��H2S���ʴ�Ϊ��HF��HCl��H2S��
��5���ܶ�Ӧ���⻯��ΪNH3��Ϊ���ۻ���������ʽΪ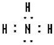 ����Һˮ����һˮ�ϰ������������ֻ��OH-����Һ�Լ��ԣ�ˮ��Һ��pH��7��
����Һˮ����һˮ�ϰ������������ֻ��OH-����Һ�Լ��ԣ�ˮ��Һ��pH��7��
�ʴ�Ϊ��NH3��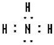 ������
������
��6���ǽ�����Cl��S����Ӧ����ۺ����������ΪHClO4��H2SO4���ʴ�Ϊ��HClO4��H2SO4��
��7��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��Na��ԭ�������������Ӱ뾶С�������Ӱ뾶ΪF-��Na+���ʴ�Ϊ��F-��Na+��
��8�������Ե���������Ϊ������������NaOH��Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ϊ��Ƶ���㣬�漰Ԫ�ص�λ�á�Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ�ȣ���Ŀ�ѶȲ���ע��Ԫ�صĽ����ԡ��ǽ����ԱȽϼ�������֪ʶ���ۺ�Ӧ�ã�

| A�� | ��ҵ����ˮ����NO2�������3NO2+H2O=2HNO3+NO | |
| B�� | �ð�ˮ��ȥ��ҵԭ���Ȼ���е��Ȼ������ʣ�Fe3++3OH-=Fe��OH��3�� | |
| C�� | ����ʯ�Ҵ���й©��Һ�ȣ�2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O | |
| D�� | ��������ȥˮ�е����������Al3++3H2O?Al��OH��3�����壩+3H+ |
| A�� | 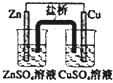 ��װ���У������е�K+����CuSO4��Һ | |
| B�� | 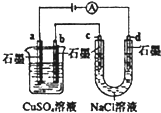 ��ͼװ����b������6.4g����ʱ��d������2.24LH2 | |
| C�� | 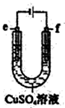 ����ͼװ�þ���ͭʱ��f��Ϊ��ͭ | |
| D�� | 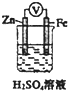 ��ͼװ���е����ص�����Zn����Fe��Fe�����д����������� |
| A�� | ԭ�Ӱ뾶 Na��Mg��Al | B�� | ���� H2SiO3��H2CO3��H2SO4 | ||
| C�� | �ȶ��� HF��HCl��HBr | D�� | ���� NaOH��Mg��OH��2��Al��OH��3 |
| A�� | ��ϩ�������� | B�� | ���ᡢ������� | C�� | ��ϩ��1-��ϩ | D�� | ��ȩ��������� |
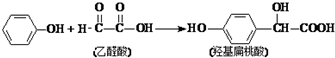
| A�� | �÷�Ӧ��ȡ����Ӧ | |
| B�� | ���Ӻ��ǻ�����������FeCl3��Һ������ɫ | |
| C�� | ��ȩ����H2�ӳɵIJ������ڴ����������γɸ߷��ӻ����� | |
| D�� | 1mol�ǻ�����������3mol NaOH��Ӧ |
| A�� | c��OH-��=c��Na+��+c��H+�� | |
| B�� | ����Һ�е�c��OH-��=1.0x10-3mol•L-1 | |
| C�� | ��ˮϡ��104��������Һ�Լ��� | |
| D�� | ��pH=3��HF��Һ��������������Һ��c��Na+��=c ��F-��+c��HF�� |
